Here are the essential concepts you must grasp in order to answer the question correctly.
Oxidation of Alcohols
Oxidation of alcohols involves the conversion of alcohols into carbonyl compounds, such as aldehydes or ketones, through the removal of hydrogen atoms. Primary alcohols typically oxidize to aldehydes, while secondary alcohols oxidize to ketones. This process is crucial in organic chemistry and is often facilitated by oxidizing agents like potassium dichromate or PCC (pyridinium chlorochromate).
Recommended video:
Alcohol Reactions: Oxidation Concept 1
Types of Carbonyl Compounds
Carbonyl compounds include aldehydes and ketones, characterized by the presence of a carbonyl group (C=O). Aldehydes have the carbonyl group at the end of the carbon chain, while ketones have it within the chain. Understanding the structural differences between these compounds is essential for determining the appropriate oxidation pathway for different alcohols.
Recommended video:
Types of Radiation Concept 2
Reactivity of Alcohols
The reactivity of alcohols during oxidation depends on their classification as primary, secondary, or tertiary. Primary alcohols can be oxidized to aldehydes and further to carboxylic acids, while secondary alcohols yield ketones. Tertiary alcohols, however, do not oxidize easily due to the lack of hydrogen atoms on the carbon bearing the hydroxyl group, making it important to identify the type of alcohol when predicting the product of oxidation.
Recommended video:
Alcohol Classification Concept 2
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution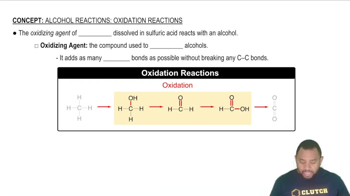



 1:49m
1:49m