Here are the essential concepts you must grasp in order to answer the question correctly.
Melting
Melting is the process where a solid turns into a liquid due to an increase in temperature. This occurs when the particles in a solid gain enough energy to overcome the forces holding them together, allowing them to move freely as a liquid. An example of melting is ice transforming into water when heated.
Recommended video:
Physical Properties of Fatty Acids Concept 2
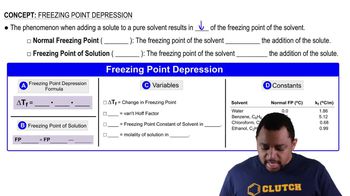
Freezing
Freezing is the transition of a liquid into a solid as it loses heat energy. During this process, the particles in the liquid slow down and arrange themselves into a fixed structure, forming a solid. An example of freezing is water turning into ice when the temperature drops below 0°C.
Recommended video:
Freezing Point Depression Concept 1
Phase Change
A phase change refers to the transformation of a substance from one state of matter to another, such as solid, liquid, or gas. These changes are driven by variations in temperature and pressure, affecting the energy and arrangement of particles. Understanding phase changes is crucial for identifying processes like melting, freezing, sublimation, and deposition.
Recommended video:
Physical & Chemical Changes
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution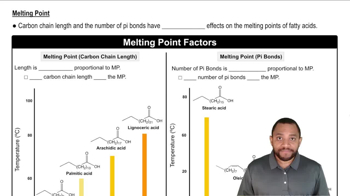


 1:35m
1:35m