Here are the essential concepts you must grasp in order to answer the question correctly.
Saturated Fatty Acids
Saturated fatty acids are types of fats that have no double bonds between the carbon atoms in their hydrocarbon chain. This means that each carbon atom is fully 'saturated' with hydrogen atoms. The absence of double bonds allows these fatty acids to pack closely together, resulting in a straight-chain structure, which is typically solid at room temperature.
Recommended video:
Molecular Structure
The molecular structure of a fatty acid refers to the arrangement of its carbon and hydrogen atoms. In an 18-carbon saturated fatty acid, the carbon atoms are connected in a linear fashion, forming a straight chain. This structural configuration is crucial for determining the physical properties of the fatty acid, such as its melting point and state at room temperature.
Recommended video:
Molecular Models Example 1
Straight-chain vs. Bent Molecules
Straight-chain molecules have a linear arrangement of atoms, allowing for tight packing and stability, while bent molecules have one or more double bonds that introduce kinks in the chain. In the case of an 18-carbon saturated fatty acid, the absence of double bonds results in a straight-chain configuration, distinguishing it from unsaturated fatty acids, which are bent due to their double bonds.
Recommended video:
Intro to Electron Transport Chain Example 1
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution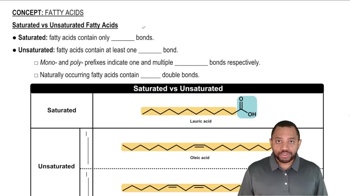



 2:0m
2:0m