Here are the essential concepts you must grasp in order to answer the question correctly.
Physical Change
A physical change refers to a transformation that alters the form or appearance of a substance without changing its chemical composition. Examples include changes in state, such as melting ice into water or dissolving sugar in water. These changes are usually reversible, meaning the original substance can be recovered.
Recommended video:
Physical and Chemical Changes
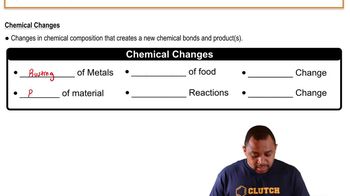
Chemical Change
A chemical change involves a process where one or more substances are transformed into different substances with distinct chemical properties. This can include reactions such as rusting iron or burning wood. Chemical changes are typically irreversible under normal conditions, as new substances are formed that cannot easily revert to their original forms.
Recommended video:
Physical & Chemical Changes
Indicators of Change
Indicators of physical and chemical changes help distinguish between the two. Physical changes may be indicated by changes in state, shape, or size, while chemical changes often involve color changes, gas production, or temperature changes. Recognizing these indicators is essential for identifying the type of change occurring in a substance.
Recommended video:
Physical & Chemical Changes
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 1:41m
1:41m