Here are the essential concepts you must grasp in order to answer the question correctly.
Dehydrogenation
Dehydrogenation is a chemical reaction that involves the removal of hydrogen atoms from a molecule. This process typically results in the oxidation of the substrate, as it loses electrons along with the hydrogen. Dehydrogenation is crucial in metabolic pathways, particularly in cellular respiration, where it helps convert energy stored in nutrients into usable forms.
FAD as a Coenzyme
FAD (flavin adenine dinucleotide) is a coenzyme that plays a vital role in various biological oxidation-reduction reactions. It acts as an electron carrier, accepting electrons during the dehydrogenation process. When FAD accepts electrons and protons, it is reduced to FADH2, which can later donate these electrons in the electron transport chain to generate ATP.
Recommended video:
Intro to Coenzymes Example 1
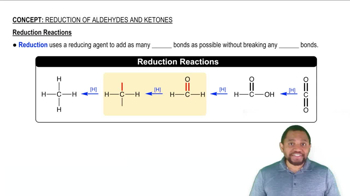
Oxidation-Reduction Reactions
Oxidation-reduction (redox) reactions are chemical processes that involve the transfer of electrons between two substances. Oxidation refers to the loss of electrons, while reduction refers to the gain of electrons. In the context of dehydrogenation, when a molecule is dehydrogenated, it is oxidized, and FAD is reduced as it accepts the electrons released during this process.
Recommended video:
Reduction Reactions Concept 1
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution

 2:2m
2:2m