Here are the essential concepts you must grasp in order to answer the question correctly.
Lewis Symbols
Lewis symbols, also known as Lewis dot diagrams, represent the valence electrons of an atom as dots around the element's chemical symbol. This visual representation helps in understanding how atoms bond with each other, as the dots indicate the number of electrons available for bonding. For example, in gallium (Ga), the Lewis symbol would show three dots, corresponding to its three valence electrons.
Recommended video:
Lewis Dot Symbols (Simplified) Concept 2
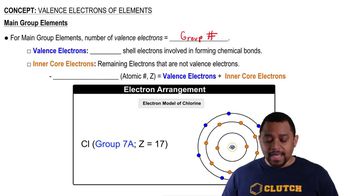
Valence Electrons
Valence electrons are the electrons in the outermost shell of an atom and are crucial for determining how an element interacts and bonds with others. The number of valence electrons influences an element's chemical properties and reactivity. Gallium, with an atomic number of 31, has three valence electrons, which play a significant role in its bonding behavior.
Recommended video:
Valence Electrons of Elements (Simplified) Concept 1
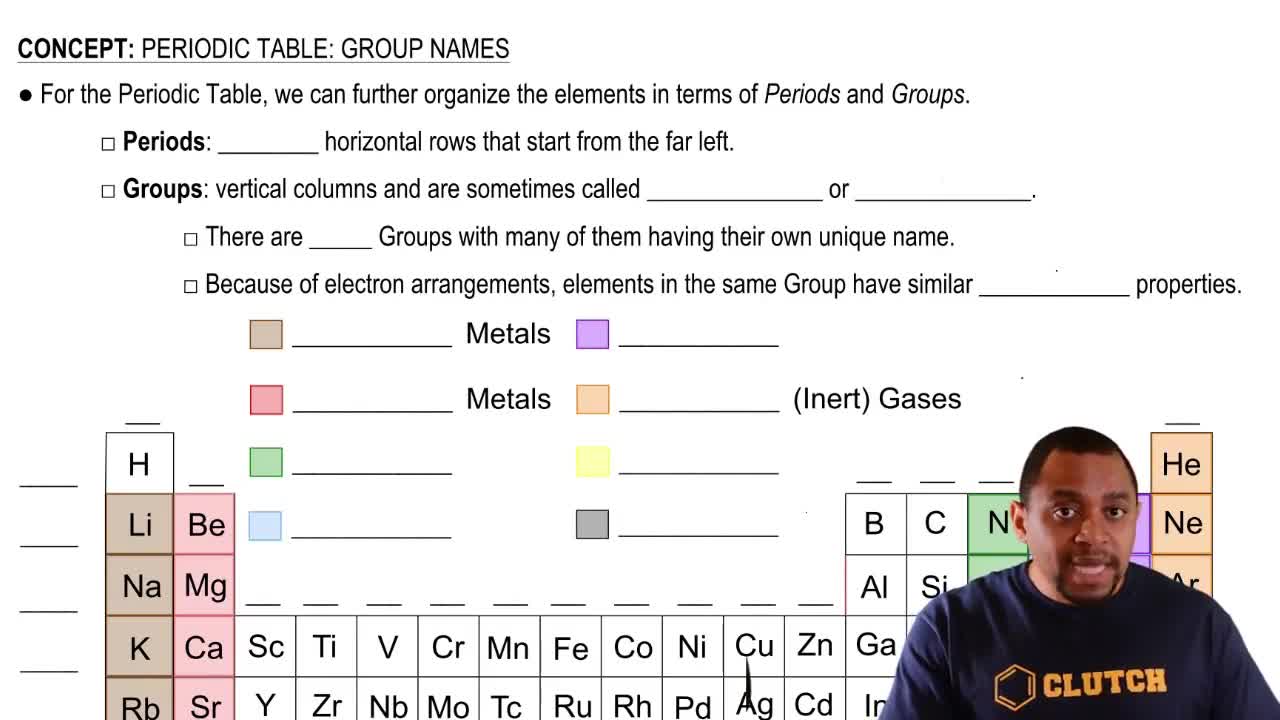
Group Number in the Periodic Table
The group number in the periodic table indicates the column in which an element is located and reflects the number of valence electrons in its outer shell. Elements in the same group typically exhibit similar chemical properties due to their comparable electron configurations. Gallium is located in Group 13, which signifies that it has three valence electrons, aligning with its Lewis symbol representation.
Recommended video:
Periodic Table: Group Names
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution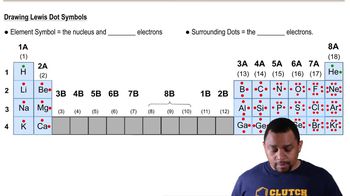

 0:52m
0:52m