Here are the essential concepts you must grasp in order to answer the question correctly.
Exothermic vs. Endothermic Reactions
Exothermic reactions release energy, usually in the form of heat, to the surroundings, resulting in a temperature increase. In contrast, endothermic reactions absorb energy from the surroundings, leading to a temperature decrease. The sign of the enthalpy change (∆H) indicates the nature of the reaction: a positive ∆H signifies an endothermic process, while a negative ∆H indicates an exothermic process.
Recommended video:
Endothermic & Exothermic Reactions
Enthalpy Change (∆H)
Enthalpy change (∆H) is a measure of the total heat content of a system during a chemical reaction. It reflects the energy absorbed or released when reactants are converted to products. In this case, a positive ∆H of +69 kcal/mol indicates that the reaction requires energy input, confirming that it is endothermic.
Recommended video:
Physical & Chemical Changes
Equilibrium Constant (K)
The equilibrium constant (K) quantifies the ratio of the concentrations of products to reactants at equilibrium for a reversible reaction. A very small K value, such as 2.68 x 10^-29, suggests that at equilibrium, the reactants are favored over the products, indicating that the formation of ozone (O3) from oxygen (O2) is not favored under standard conditions.
Recommended video:
The Equilibrium Constant Concept 1
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution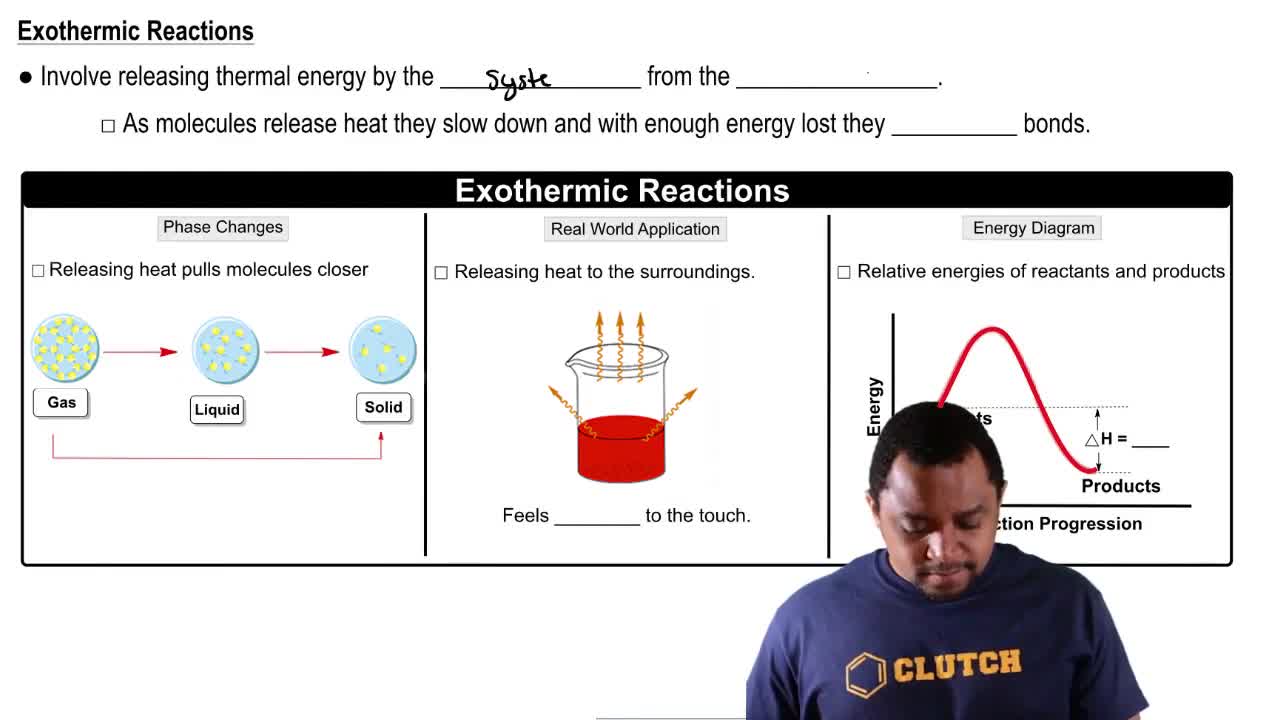



 2:30m
2:30m