Here are the essential concepts you must grasp in order to answer the question correctly.
Chemical Reaction Types
Understanding the type of chemical reaction is crucial for balancing equations. In this case, the reaction involves an acid (HBr) reacting with a carbonate (ZnCO₃), which typically results in the formation of a salt, water, and carbon dioxide. Recognizing this helps predict the products and balance the equation correctly.
Recommended video:
Types of Chemical Reactions Concept 1
Balancing Chemical Equations
Balancing chemical equations is essential to ensure that the law of conservation of mass is upheld, meaning the number of atoms for each element must be the same on both sides of the equation. This involves adjusting coefficients in front of compounds to achieve equal numbers of each type of atom, which is a fundamental skill in chemistry.
Recommended video:
Balancing Chemical Equations (Simplified) Concept 1
Product Formation in Acid-Base Reactions
In acid-base reactions, such as the one between HBr and ZnCO₃, the products typically include a salt and water, along with carbon dioxide when a carbonate is involved. Recognizing the expected products helps in writing and balancing the equation accurately, as it guides the identification of what will be formed during the reaction.
Recommended video:
Acid-Base Reactions Concept 1
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution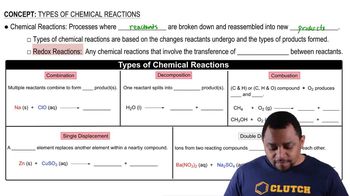



 3:08m
3:08m