Textbook Question
Which assumptions of the kinetic–molecular theory explain the behavior of gases described by Gay-Lussac's law? Explain your answer.
1156
views

 Verified step by step guidance
Verified step by step guidance
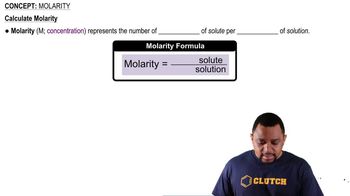


Which assumptions of the kinetic–molecular theory explain the behavior of gases described by Gay-Lussac's law? Explain your answer.
A gas has a volume of 2.84 L at 1.00 atm and 0 °C. At what temperature does it have a volume of 7.50 L at 520 mmHg?
Explain Avogadro's law using the kinetic–molecular theory of gases.
What is the mass of CH4 in a sample that occupies a volume of 16.5 L at STP?
How does the ideal gas law differ from the combined gas law?
Which sample contains more molecules: 2.0 L of Cl2 at STP or 3.0 L of CH4 at 300 K and 1150 mmHg? Which sample weighs more?