Here are the essential concepts you must grasp in order to answer the question correctly.
Phosphonium Ion
A phosphonium ion, such as PH₄⁺, is a positively charged species formed when phosphine (PH₃) reacts with an acid. In this process, the phosphine donates a lone pair of electrons to the proton (H⁺) from the acid, resulting in a stable cation. Understanding the formation of phosphonium ions is crucial for predicting their properties and reactivity.
Recommended video:
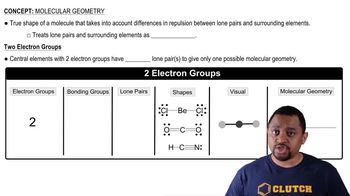
Molecular Geometry
Molecular geometry refers to the three-dimensional arrangement of atoms within a molecule. It is determined by the number of bonding pairs and lone pairs of electrons around the central atom, following the principles of VSEPR (Valence Shell Electron Pair Repulsion) theory. For PH₄⁺, the geometry can be predicted based on its electron pair arrangement.
Recommended video:
Molecular Geometry (Simplified) Concept 1
VSEPR Theory
VSEPR (Valence Shell Electron Pair Repulsion) theory is a model used to predict the shape of molecules based on the repulsion between electron pairs surrounding a central atom. According to this theory, electron pairs will arrange themselves to minimize repulsion, leading to specific molecular geometries. For PH₄⁺, the presence of four bonding pairs suggests a tetrahedral geometry.
Recommended video: