Here are the essential concepts you must grasp in order to answer the question correctly.
pH Scale
The pH scale measures the acidity or basicity of a solution, ranging from 0 to 14. A pH of 7 is considered neutral, while values below 7 indicate acidity and above 7 indicate basicity. The scale is logarithmic, meaning each whole number change represents a tenfold change in hydrogen ion concentration.
Recommended video:
Strong Acids
Strong acids, like HBr, completely dissociate in water, releasing all their hydrogen ions (H+). This complete dissociation means that the concentration of hydrogen ions in a strong acid solution is equal to the concentration of the acid itself. For a 0.100 M HBr solution, the concentration of H+ ions is also 0.100 M.
Recommended video:
Calculating pH
To calculate the pH of a solution, the formula pH = -log[H+] is used, where [H+] is the concentration of hydrogen ions. For a 0.100 M HBr solution, since it fully dissociates, the pH can be calculated as pH = -log(0.100), resulting in a pH of 1.00, indicating a strongly acidic solution.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution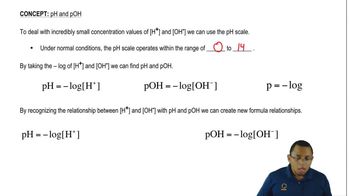



 2:39m
2:39m