Here are the essential concepts you must grasp in order to answer the question correctly.
Physical Property
A physical property is a characteristic of a substance that can be observed or measured without changing its chemical composition. Examples include color, odor, melting point, and density. In the context of apple slices turning brown, the initial appearance of the slices is a physical property that can be observed before any chemical changes occur.
Recommended video:
Physical Properties Concept
Chemical Property
A chemical property describes a substance's ability to undergo a specific chemical change. This includes reactions with other substances, such as oxidation or combustion. The browning of apple slices is a chemical property, as it involves the reaction of phenolic compounds in the apples with oxygen in the air, leading to the formation of brown pigments.
Recommended video:
Chemical Properties Example 1
Oxidation Reaction
An oxidation reaction is a type of chemical reaction where a substance loses electrons, often involving the reaction with oxygen. In the case of apple slices, the exposure to air triggers oxidation, causing the phenolic compounds to oxidize and produce melanin, which results in the browning effect. This process is a key example of how environmental factors can influence chemical properties.
Recommended video:
Alcohol Reactions Oxidation Reactions Example 1
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: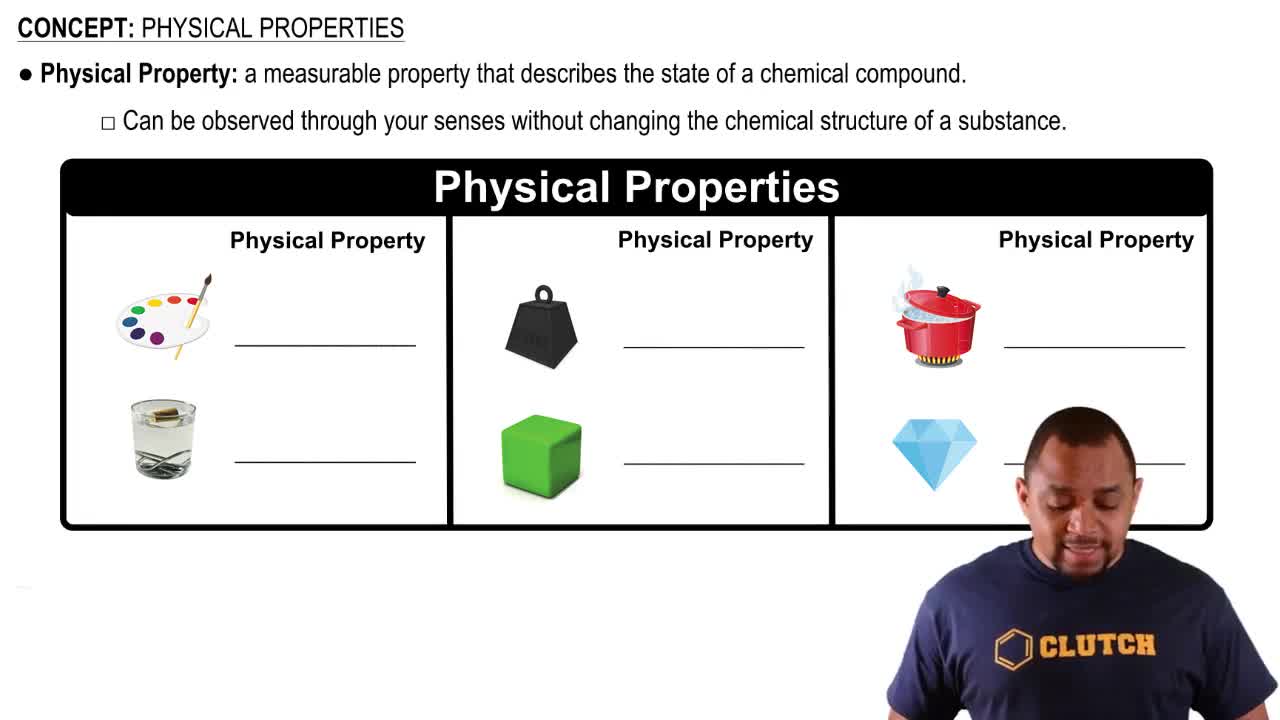



 2:54m
2:54m