Here are the essential concepts you must grasp in order to answer the question correctly.
Electronegativity
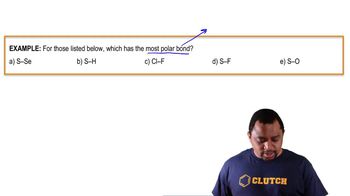
Electronegativity is a measure of an atom's ability to attract and hold onto electrons in a chemical bond. It is a key factor in determining the nature of the bond formed between two atoms. The greater the difference in electronegativity between two atoms, the more likely the bond will be ionic, as one atom will attract the shared electrons more strongly than the other.
Recommended video:
Dipole Moment (Simplified) Concept 1
Ionic vs. Covalent Bonds
Ionic bonds occur when electrons are transferred from one atom to another, resulting in the formation of charged ions that attract each other. In contrast, covalent bonds involve the sharing of electrons between atoms. The type of bond formed depends on the electronegativity difference: a large difference typically leads to ionic bonds, while a small difference results in covalent bonds.
Recommended video:
Electronegativity Difference
The electronegativity difference between two atoms can be used to predict the bond type. Generally, a difference of 1.7 or greater indicates an ionic bond, while a difference of less than 1.7 suggests a covalent bond. In the case of calcium (Ca) and chlorine (Cl), the significant electronegativity difference suggests that the bond formed between them would be largely ionic.
Recommended video:
Dipole Moment (Simplified) Example 3
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 1:51m
1:51m