Here are the essential concepts you must grasp in order to answer the question correctly.
Coupled Reactions
Coupled reactions refer to a process where two chemical reactions occur simultaneously, with the energy released from one reaction driving the other. This is often seen in biological systems, where exergonic reactions (releasing energy) are paired with endergonic reactions (requiring energy) to facilitate essential cellular processes.
Recommended video:
Coupled Reactions Concept 3
Thermodynamics
Thermodynamics is the study of energy transformations and the laws governing these processes. In the context of coupled reactions, the first law of thermodynamics states that energy cannot be created or destroyed, which means the energy released from one reaction can be harnessed to power another, maintaining the overall energy balance in a system.
Recommended video:
First Law of Thermodynamics
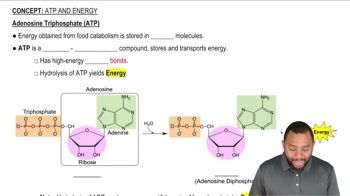
ATP Hydrolysis
ATP hydrolysis is a specific example of a coupled reaction where the breakdown of adenosine triphosphate (ATP) releases energy that is used to drive various cellular processes. This reaction is crucial in metabolism, as it provides the necessary energy for endergonic reactions, illustrating the concept of coupling in biological systems.
Recommended video:
Adenosine Triphosphate (ATP) Concept 2
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: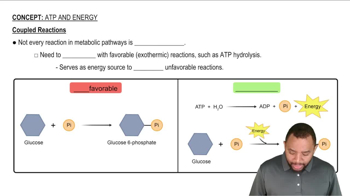


 1:46m
1:46m