Here are the essential concepts you must grasp in order to answer the question correctly.
pH Scale
The pH scale measures the acidity or basicity of a solution, ranging from 0 to 14. A pH of 7 is considered neutral, while values below 7 indicate acidity and values above 7 indicate alkalinity. The scale is logarithmic, meaning each whole number change represents a tenfold change in hydrogen ion concentration.
Recommended video:
Hydroxide Ion Concentration
Hydroxide ions (OH⁻) are crucial in determining the basicity of a solution. The concentration of hydroxide ions can be used to calculate the pOH of a solution, which is related to pH through the equation pH + pOH = 14. Understanding the relationship between hydroxide and hydrogen ions is essential for pH calculations.
Recommended video:
Percent Concentrations Concept 1
Ion Product of Water
The ion product of water (Kw) is the equilibrium constant for the self-ionization of water, defined as Kw = [H⁺][OH⁻] = 1 x 10⁻¹⁴ at 25°C. This relationship allows for the calculation of pH from hydroxide ion concentration, as knowing [OH⁻] enables the determination of [H⁺] and subsequently the pH of the solution.
Recommended video:
Solubility Product Constant (Ksp) Concept 2
 Verified Solution
Verified Solution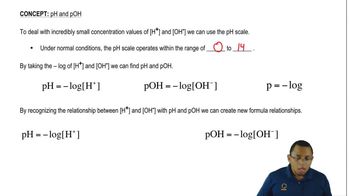


 1:31m
1:31m