Here are the essential concepts you must grasp in order to answer the question correctly.
Molecular Formulas
A molecular formula represents the number and types of atoms in a molecule. It is expressed using chemical symbols and subscripts, indicating how many of each atom are present. For example, in CH₂Cl, 'C' stands for carbon, 'H' for hydrogen, and 'Cl' for chlorine, with the subscripts showing the quantity of each atom.
Recommended video:
Valency and Bonding
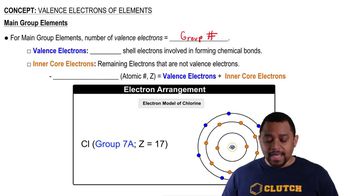
Valency refers to the ability of an atom to bond with other atoms, determined by the number of electrons in its outer shell. Understanding valency is crucial for predicting how atoms combine to form molecules. For instance, carbon typically has a valency of four, allowing it to form four bonds, while chlorine has a valency of one.
Recommended video:
Valence Electrons of Elements (Simplified) Concept 1
Common Chemical Notation
Chemical notation is a system of symbols used to represent chemical substances and their interactions. It includes not only molecular formulas but also structural formulas that depict how atoms are arranged. Familiarity with this notation helps in interpreting and writing chemical formulas accurately, which is essential for identifying the correct formulas for the given molecules.
Recommended video: