Textbook Question
What is the mass of CH4 in a sample that occupies a volume of 16.5 L at STP?
2257
views

 Verified step by step guidance
Verified step by step guidance
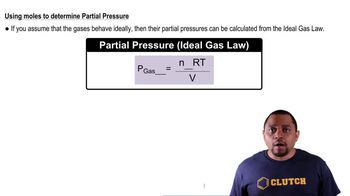


What is the mass of CH4 in a sample that occupies a volume of 16.5 L at STP?
How does the ideal gas law differ from the combined gas law?
Which sample contains more molecules: 2.0 L of Cl2 at STP or 3.0 L of CH4 at 300 K and 1150 mmHg? Which sample weighs more?
What is a liquid's heat of vaporization?
What is the effect of pressure on a liquid's boiling point?
List three kinds of crystalline solids, and give an example of each.