Here are the essential concepts you must grasp in order to answer the question correctly.
Partial Pressure
Partial pressure refers to the pressure exerted by a single component of a gas mixture. According to Dalton's Law of Partial Pressures, the total pressure of a gas mixture is equal to the sum of the partial pressures of each individual gas present. This concept is crucial in understanding gas behavior in various scientific fields, including chemistry and physics.
Recommended video:
Dalton's Law: Partial Pressure (Simplified) Concept 2
Dalton's Law of Partial Pressures
Dalton's Law states that in a mixture of non-reacting gases, the total pressure is the sum of the partial pressures of each gas. This law allows for the calculation of the contribution of each gas to the overall pressure, which is essential in applications such as respiratory physiology and atmospheric science.
Recommended video:
Dalton's Law: Partial Pressure (Simplified) Concept 3
Gas Mixtures
Gas mixtures consist of two or more gases that can coexist without reacting chemically. Understanding the behavior of gas mixtures, including how each gas contributes to the total pressure, is fundamental in fields like environmental science, engineering, and medicine, particularly in understanding how gases interact in different environments.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution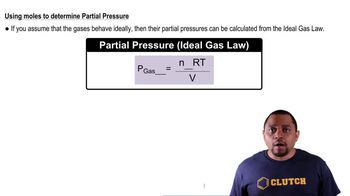



 0:44m
0:44m