Here are the essential concepts you must grasp in order to answer the question correctly.
Lewis Symbols
Lewis symbols are a way to represent the valence electrons of an atom using dots around the element's symbol. Each dot corresponds to a valence electron, and the arrangement helps visualize how atoms bond with each other. For representative elements, the number of dots typically corresponds to the group number in the periodic table.
Recommended video:
Lewis Dot Symbols (Simplified) Concept 2
Ion Formation
Ions are charged particles that form when atoms gain or lose electrons. A cation is formed when an atom loses electrons, resulting in a positive charge, while an anion is formed when an atom gains electrons, resulting in a negative charge. The symbol Y³⁻ indicates that the element has gained three electrons, making it an anion.
Recommended video:
Amide Formation Concept 1
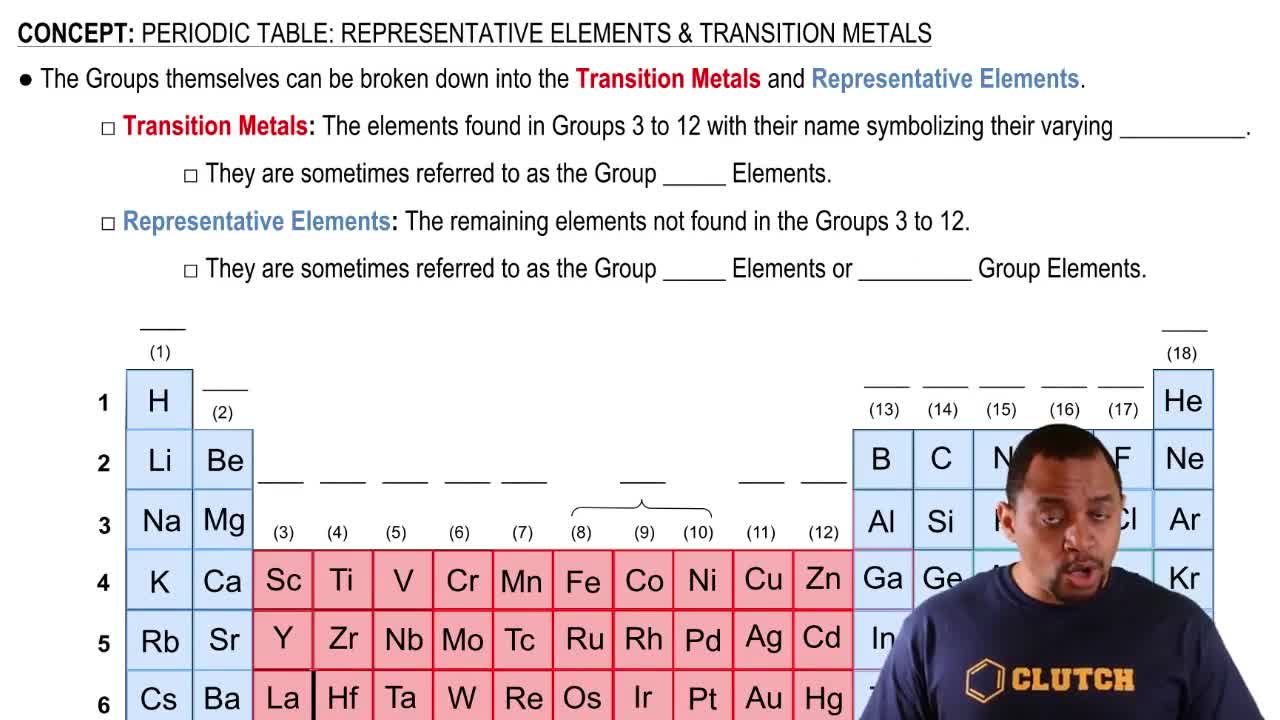
Representative Elements
Representative elements are found in groups 1, 2, and 13-18 of the periodic table and include metals, nonmetals, and metalloids. These elements exhibit a wide range of chemical properties and typically follow predictable patterns in their electron configurations. Understanding their behavior is crucial for predicting how they will form ions and bonds.
Recommended video:
Periodic Table: Representative Elements & Transition Metals
 Verified Solution
Verified Solution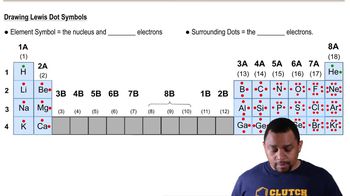

 0:52m
0:52m
