Here are the essential concepts you must grasp in order to answer the question correctly.
Condensation Reaction
A condensation reaction is a chemical process where two molecules combine to form a larger molecule, releasing a small molecule, often water, as a byproduct. This type of reaction is crucial in forming complex organic compounds, such as proteins and polysaccharides, from simpler monomers. Understanding this reaction is essential for analyzing biochemical pathways in allied health.
Recommended video:
Condensed Formula Concept 1
Hydrolysis Reaction
Hydrolysis is the process of breaking down a compound by adding water, resulting in the separation of larger molecules into smaller units. This reaction is vital in digestion, where complex carbohydrates, proteins, and fats are broken down into their monomeric forms for absorption. Recognizing hydrolysis is important for understanding metabolic processes in the body.
Recommended video:
Triacylglycerol Reactions: Hydrolysis Concept 1
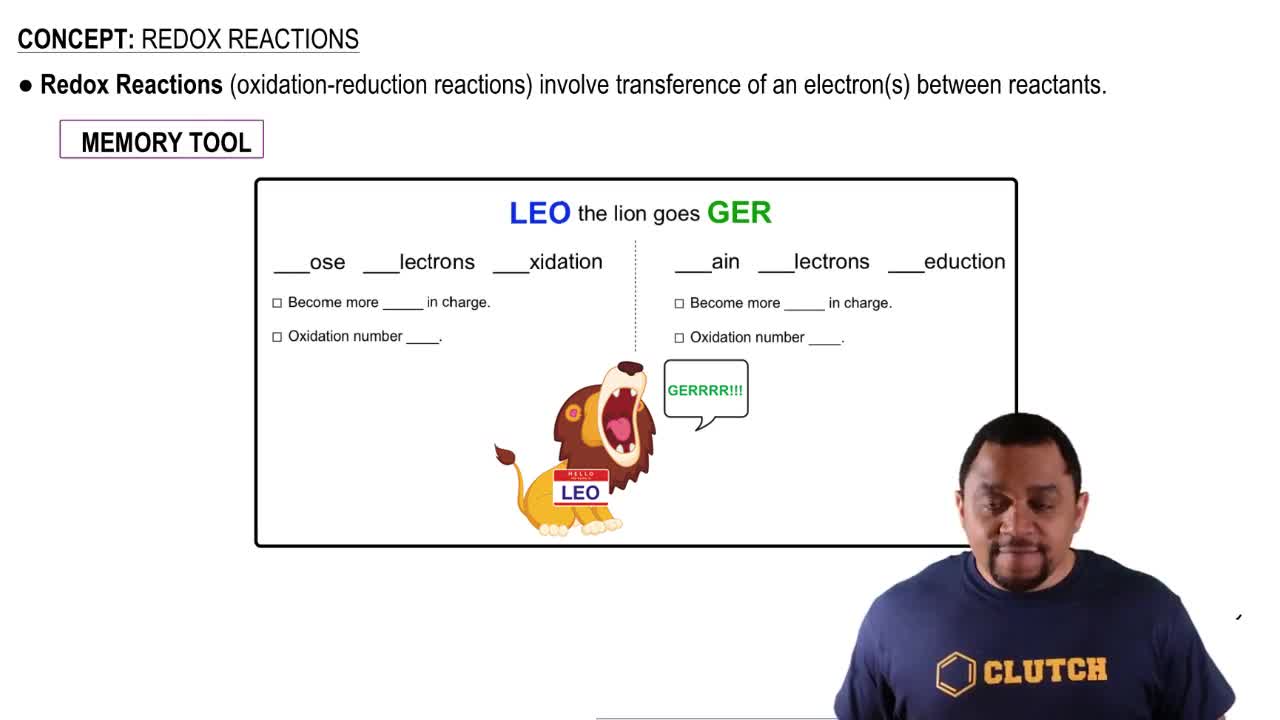
Oxidation-Reduction (Redox) Reactions
Oxidation-reduction reactions involve the transfer of electrons between substances, leading to changes in their oxidation states. Oxidation refers to the loss of electrons, while reduction refers to the gain of electrons. These reactions are fundamental in cellular respiration and energy production, making them critical for comprehending metabolic functions in allied health.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 3:12m
3:12m