Here are the essential concepts you must grasp in order to answer the question correctly.
Atomic Mass Unit (amu)
The atomic mass unit (amu) is a standard unit of mass used to express atomic and molecular weights. It is defined as one twelfth of the mass of a carbon-12 atom, which has six protons and six neutrons. This unit allows for the comparison of the mass of different atoms and molecules on a relative scale.
Recommended video:
Atomic Mass (Conceptual) Concept 1
Carbon-12 Isotope
Carbon-12 is a stable isotope of carbon, consisting of six protons and six neutrons. It is the most abundant isotope of carbon and serves as the standard reference for defining the atomic mass unit. Understanding carbon-12 is crucial for grasping how atomic masses are calculated and compared.
Recommended video:
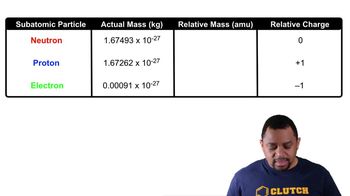
Protons and Neutrons
Protons and neutrons are subatomic particles found in the nucleus of an atom. Protons carry a positive charge, while neutrons are neutral. The number of protons determines the element's identity, while the total number of protons and neutrons gives the atomic mass. This distinction is essential for understanding atomic structure and mass calculations.
Recommended video:
Subatomic Particles (Simplified) Concept 2
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 1:25m
1:25m