Here are the essential concepts you must grasp in order to answer the question correctly.
Le Chatelier's Principle
Le Chatelier's Principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts to counteract the change. In the context of the reaction provided, adding more SO₂(g) would shift the equilibrium to the right, favoring the production of SO₃(g) to reduce the concentration of SO₂(g).
Recommended video:
The following is an endothermic reaction where Kc = 6.73 x 103.For each of the choices below predict in which direction the reaction will proceed
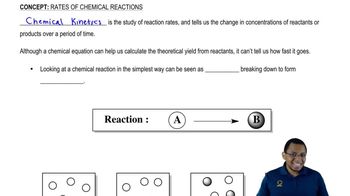
Reaction Rate
The reaction rate refers to the speed at which reactants are converted into products in a chemical reaction. It can be influenced by factors such as concentration, temperature, and the presence of catalysts. Increasing the concentration of SO₂(g) increases the likelihood of collisions between reactant molecules, thereby increasing the rate of the reaction.
Recommended video:
Rate of Reaction Concept 1
Equilibrium Constant (K)
The equilibrium constant (K) is a numerical value that expresses the ratio of the concentrations of products to reactants at equilibrium for a given reaction at a specific temperature. For the reaction 2SO₂(g) + O₂(g) ⇌ 2SO₃(g), adding SO₂(g) does not change the value of K but alters the concentrations of the reactants and products, affecting the position of equilibrium and the rate of reaction until a new equilibrium is established.
Recommended video:
The Equilibrium Constant Concept 1
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 1:07m
1:07m