Here are the essential concepts you must grasp in order to answer the question correctly.
Polar Amino Acids
Polar amino acids have side chains that are hydrophilic, meaning they can interact favorably with water and other polar substances. These amino acids typically contain functional groups such as hydroxyl (-OH), carboxyl (-COOH), or amine (-NH2) groups, which can form hydrogen bonds. Examples include serine, threonine, and asparagine, which play crucial roles in protein structure and function due to their ability to form interactions with the aqueous environment.
Recommended video:
Polar Amino Acids Concept 2
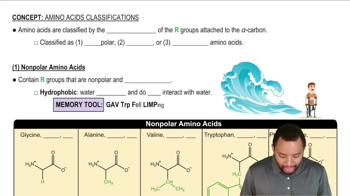
Nonpolar Amino Acids
Nonpolar amino acids possess side chains that are hydrophobic, making them less likely to interact with water. Their side chains are primarily composed of hydrocarbons, which do not form hydrogen bonds with water. Examples include alanine, valine, and leucine. These amino acids tend to cluster together in the interior of proteins, contributing to the overall stability and folding of the protein structure by minimizing exposure to the aqueous environment.
Recommended video:
Nonpolar Amino Acids Concept 1
Hydrophobic Effect
The hydrophobic effect is a key principle in biochemistry that describes how nonpolar substances tend to aggregate in aqueous solutions to minimize their exposure to water. This phenomenon is crucial for protein folding, as nonpolar amino acids will often be found in the core of proteins, away from the surrounding water. Understanding this effect helps explain the structural organization of proteins and the behavior of amino acids in biological systems.
Recommended video:
Solubility: Temperature Effect Concept 1
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 5:16m
5:16m