Here are the essential concepts you must grasp in order to answer the question correctly.
Ion Formation
Ions are charged particles that form when atoms gain or lose electrons. A cation has a positive charge due to the loss of electrons, while an anion has a negative charge from gaining electrons. In this case, Y³⁻ indicates that the element has gained three electrons, resulting in a negative charge.
Recommended video:
Amide Formation Concept 1
Periodic Table and Periods
The periodic table organizes elements by increasing atomic number and groups them into periods and groups. A period corresponds to the horizontal rows of the table, indicating the highest energy level of electrons in the elements. Period 3 includes elements with three electron shells, which is crucial for identifying the element Y.
Recommended video:
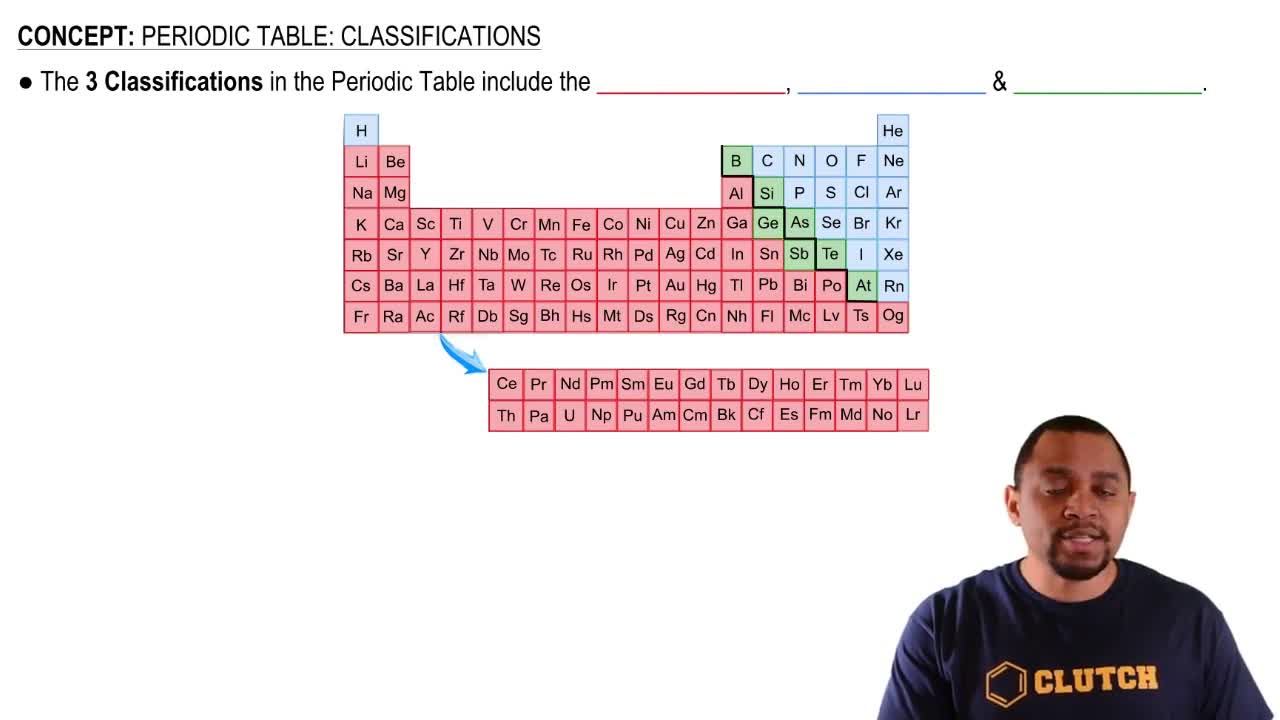
Periodic Table: Classifications
Representative Elements
Representative elements, also known as main group elements, are found in groups 1, 2, and 13-18 of the periodic table. These elements typically exhibit a wide range of chemical and physical properties and are characterized by their ability to form stable ions. Identifying Y as a representative element in Period 3 helps narrow down the possibilities to elements like phosphorus or sulfur.
Recommended video:
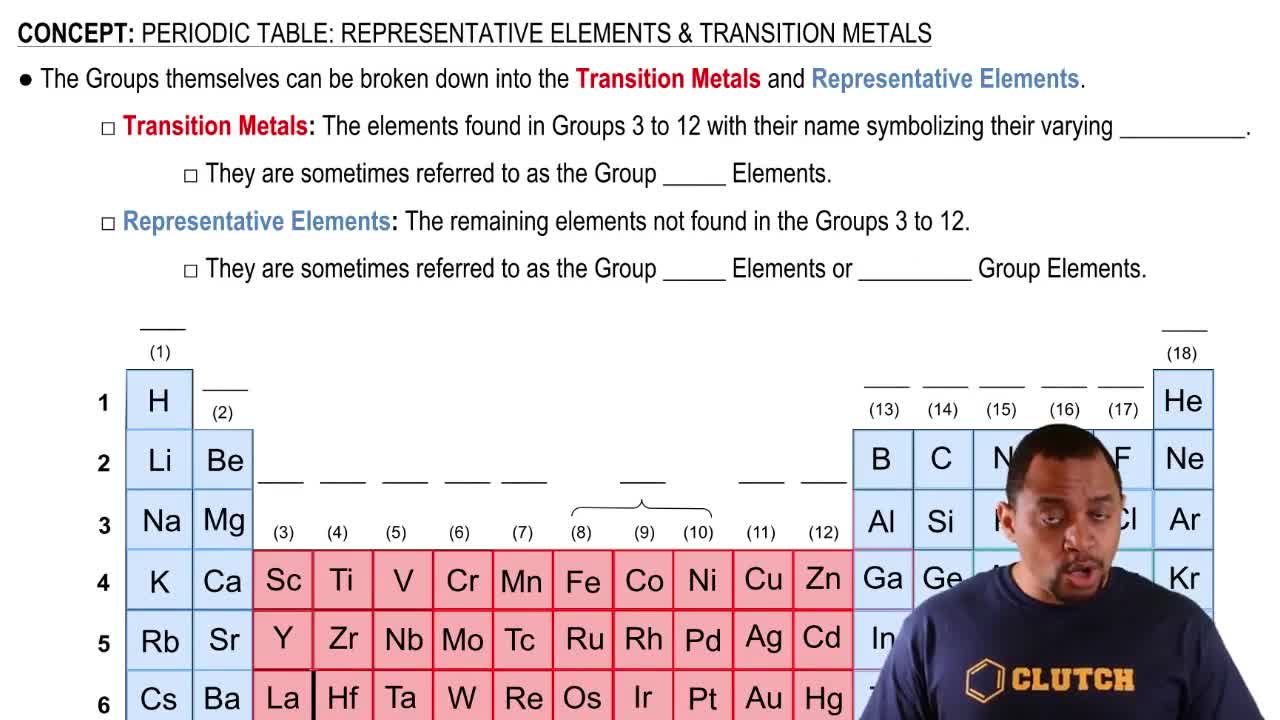
Periodic Table: Representative Elements & Transition Metals
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution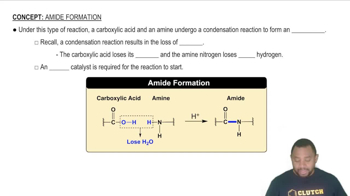

 2:15m
2:15m