Here are the essential concepts you must grasp in order to answer the question correctly.
Half-Life
Half-life is the time required for half of the radioactive nuclei in a sample to decay. It is a crucial concept in understanding radioactive decay processes, as it allows us to predict how much of a substance will remain after a certain period. In this case, the half-life of Ce-141 is 32.5 days, meaning that every 32.5 days, the activity of the sample will reduce by half.
Recommended video:
Radioactive Half-Life Concept 1
Radioactive Decay
Radioactive decay is the process by which an unstable atomic nucleus loses energy by emitting radiation. This process results in the transformation of the original element into a different element or isotope. The activity of a radioactive sample, measured in microcuries (µCi), indicates the rate of decay, which decreases over time as the material transforms into stable products.
Recommended video:
Measuring Radioactivity Concept 1
Exponential Decay Formula
The exponential decay formula describes how the quantity of a radioactive substance decreases over time. It is expressed as A(t) = A0 * (1/2)^(t/T), where A(t) is the remaining activity at time t, A0 is the initial activity, and T is the half-life. This formula is essential for calculating the initial activity of the sample based on the known activity after a specific time period.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution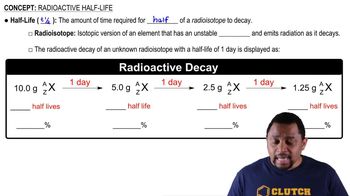



 2:09m
2:09m