Here are the essential concepts you must grasp in order to answer the question correctly.
Tollens' Test
Tollens' test is a qualitative test used to identify aldehydes and certain reducing sugars. It involves the use of Tollens' reagent, which contains silver nitrate in ammonia, that oxidizes aldehydes to carboxylic acids while reducing silver ions to metallic silver. A positive result is indicated by the formation of a silver mirror on the test container.
Recommended video:
Aldehydes vs. Alcohols
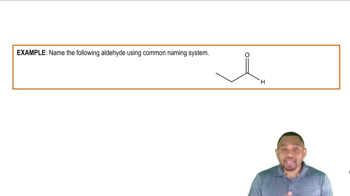
Aldehydes are organic compounds characterized by the presence of a carbonyl group (C=O) at the end of the carbon chain, making them reactive and capable of undergoing oxidation. In contrast, alcohols contain a hydroxyl group (–OH) and are generally less reactive in oxidation reactions, which is crucial for determining the outcome of the Tollens' test.
Recommended video:
Naming Aldehydes Example 2
Oxidation and Reduction
Oxidation refers to the loss of electrons or an increase in oxidation state, while reduction is the gain of electrons or a decrease in oxidation state. In the context of the Tollens' test, aldehydes are oxidized to carboxylic acids, and the silver ions in Tollens' reagent are reduced to metallic silver, demonstrating the redox nature of the reaction.
Recommended video:
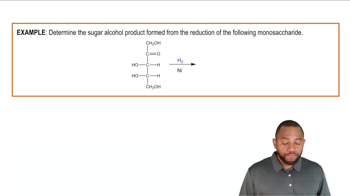
Reduction of Monosaccharides Example 1
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution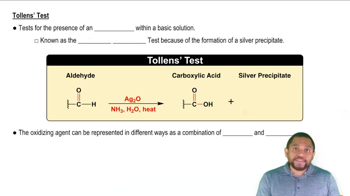

 1:57m
1:57m