Here are the essential concepts you must grasp in order to answer the question correctly.
Henry's Law
Henry's Law states that the amount of gas that dissolves in a liquid at a given temperature is directly proportional to the partial pressure of that gas above the liquid. This principle is crucial for understanding how changes in pressure affect the solubility of gases like CO₂ in water, which is essential for calculating the equilibrium concentration in the soft drink.
Recommended video:
Henry's Law Calculations Concept 1
Equilibrium
Equilibrium in a chemical context refers to the state where the rate of the forward reaction equals the rate of the reverse reaction, resulting in constant concentrations of reactants and products. In this scenario, it describes the balance between CO₂ dissolved in the soft drink and the CO₂ in the surrounding atmosphere, influencing how much gas remains in solution.
Recommended video:
The Equilibrium Constant Example 1
Parts Per Million (ppm)
Parts per million (ppm) is a unit of measurement used to describe the concentration of one substance in a million parts of another. In this question, the atmospheric concentration of CO₂ is given in ppm, which helps to convert this concentration into a partial pressure, allowing for the application of Henry's Law to determine how much CO₂ remains dissolved in the soft drink.
Recommended video:
Parts per Million (ppm) Concept 1
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution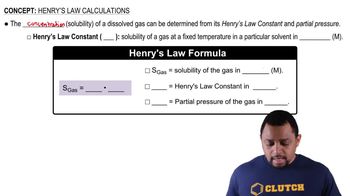



 1:41m
1:41m