Here are the essential concepts you must grasp in order to answer the question correctly.
Ion Formation
Ions are charged particles that form when atoms gain or lose electrons. A cation, like X²⁺, indicates that the atom has lost two electrons. Understanding how ions form is crucial for identifying elements based on their charge and electron configuration.
Recommended video:
Amide Formation Concept 1
Periodic Table Trends
The periodic table organizes elements by increasing atomic number and groups them based on similar properties. Elements in the same period share the same number of electron shells. Recognizing the position of an element in the periodic table helps in predicting its properties and behavior, including its ionization.
Recommended video:
Periodic Trend: Metallic Character
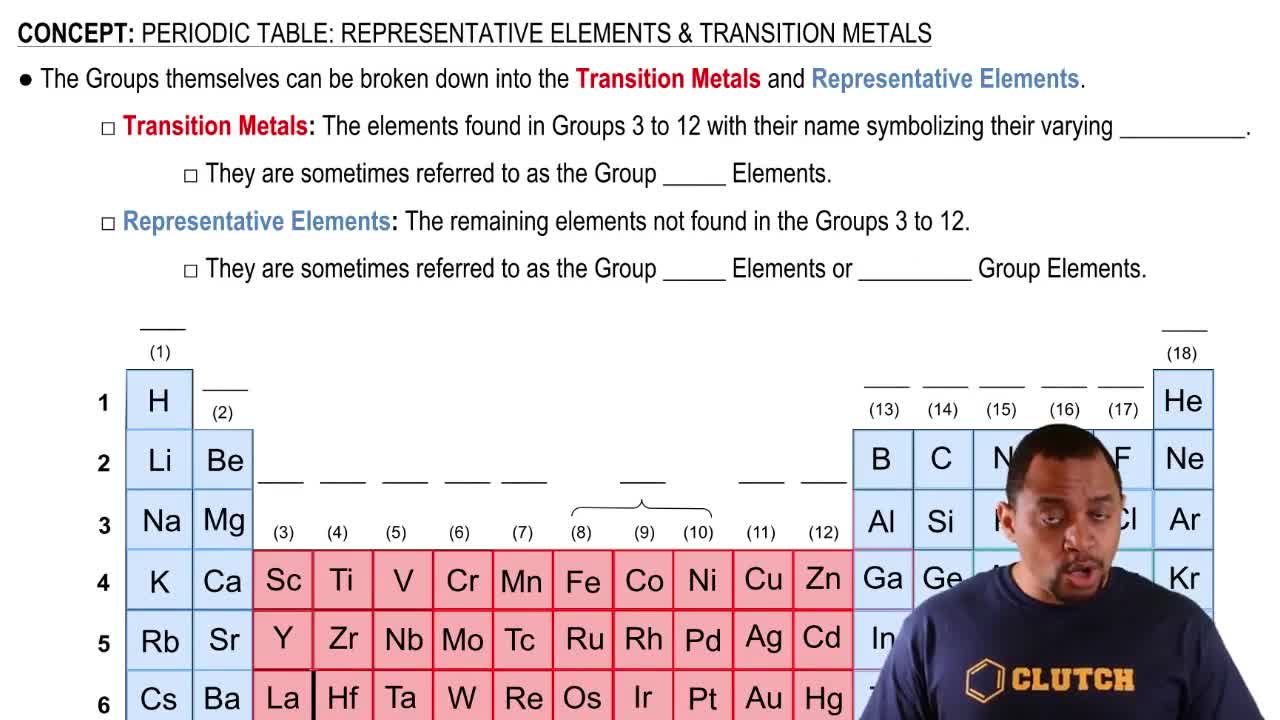
Representative Elements
Representative elements, found in groups 1, 2, and 13-18 of the periodic table, exhibit a wide range of chemical and physical properties. These elements typically follow predictable patterns in their ionization and bonding behavior. Identifying X as a representative element in Period 3 aids in determining its identity based on its common oxidation states.
Recommended video:
Periodic Table: Representative Elements & Transition Metals
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 2:15m
2:15m