Here are the essential concepts you must grasp in order to answer the question correctly.
Kinetic-Molecular Theory
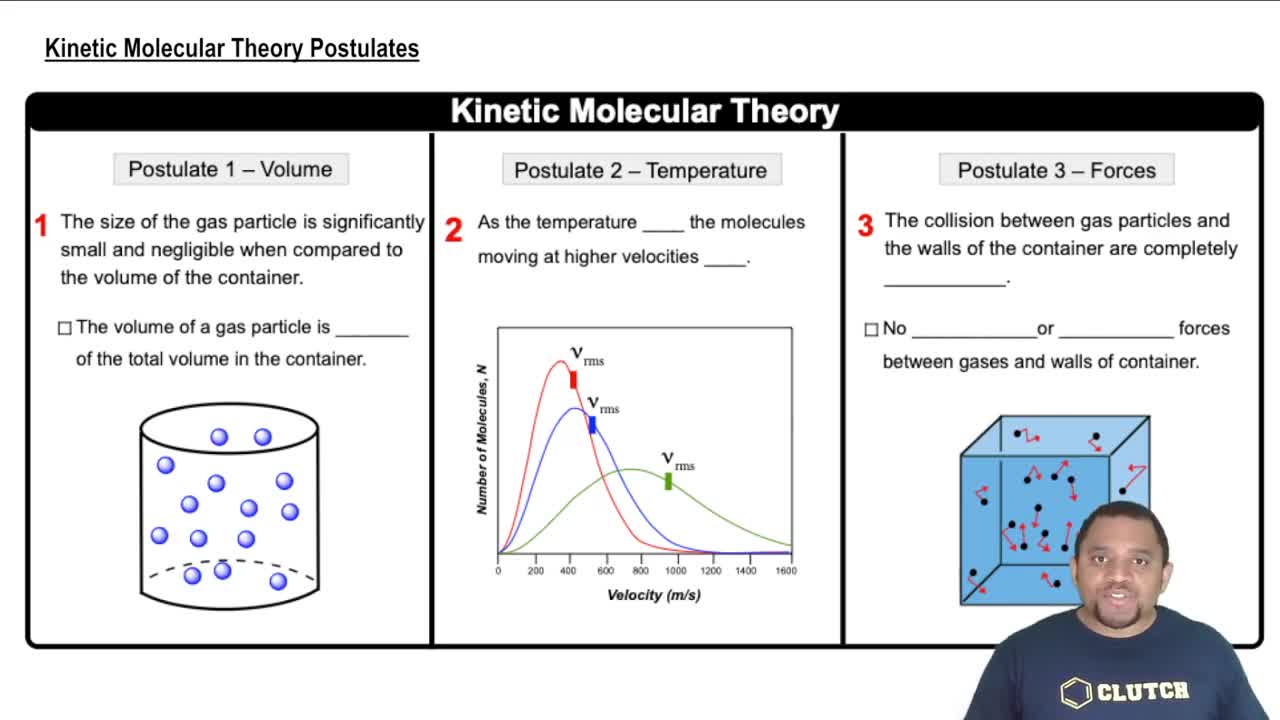
The kinetic-molecular theory posits that gas consists of a large number of small particles in constant, random motion. This theory explains gas behavior by relating temperature, pressure, and volume to the motion and interactions of these particles. It assumes that gas particles are far apart, have negligible volume, and experience elastic collisions, which are crucial for understanding gas laws.
Recommended video:
Boyle's Law
Boyle's law states that the pressure of a gas is inversely proportional to its volume when temperature is held constant. This means that as the volume of a gas decreases, its pressure increases, provided the temperature remains unchanged. This relationship can be explained through the kinetic-molecular theory, as reducing volume increases particle collisions, thereby increasing pressure.
Recommended video:
Chemistry Gas Laws: Combined Gas Law
Elastic Collisions
Elastic collisions refer to interactions between gas particles where no kinetic energy is lost. In the context of the kinetic-molecular theory, these collisions are essential for maintaining the energy and momentum of gas particles. Understanding elastic collisions helps explain how changes in volume affect pressure, as more frequent collisions at reduced volume lead to higher pressure, consistent with Boyle's law.
Recommended video:
 Verified Solution
Verified Solution


 0:58m
0:58m
