Here are the essential concepts you must grasp in order to answer the question correctly.
Half-Life
Half-life is the time required for half of a sample of a radioactive substance to decay. It is a constant property of the substance and is crucial for understanding the rate of decay. For example, if a substance has a half-life of 2.7 days, after this period, only half of the original amount remains.
Recommended video:
Radioactive Half-Life Concept 1
Decay Process
The decay process refers to the transformation of unstable atomic nuclei into more stable forms, releasing energy in the form of radiation. This process occurs at a predictable rate defined by the half-life, allowing for calculations of remaining quantities over time. Understanding this process is essential for determining how many half-lives have elapsed.
Recommended video:
Processing of Pre-mRNA Concept 1
Elapsed Time Calculation
Elapsed time calculation involves determining how many half-lives have passed based on the total time elapsed. By dividing the total time by the half-life duration, one can ascertain the number of half-lives that have occurred. In the case of Au-198 with a half-life of 2.7 days, after 5.4 days, two half-lives have elapsed.
Recommended video:
Henry's Law Calculations Example 1
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution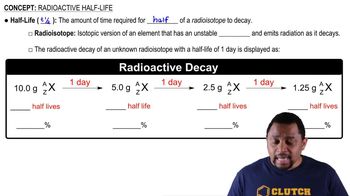



 2:09m
2:09m