Here are the essential concepts you must grasp in order to answer the question correctly.
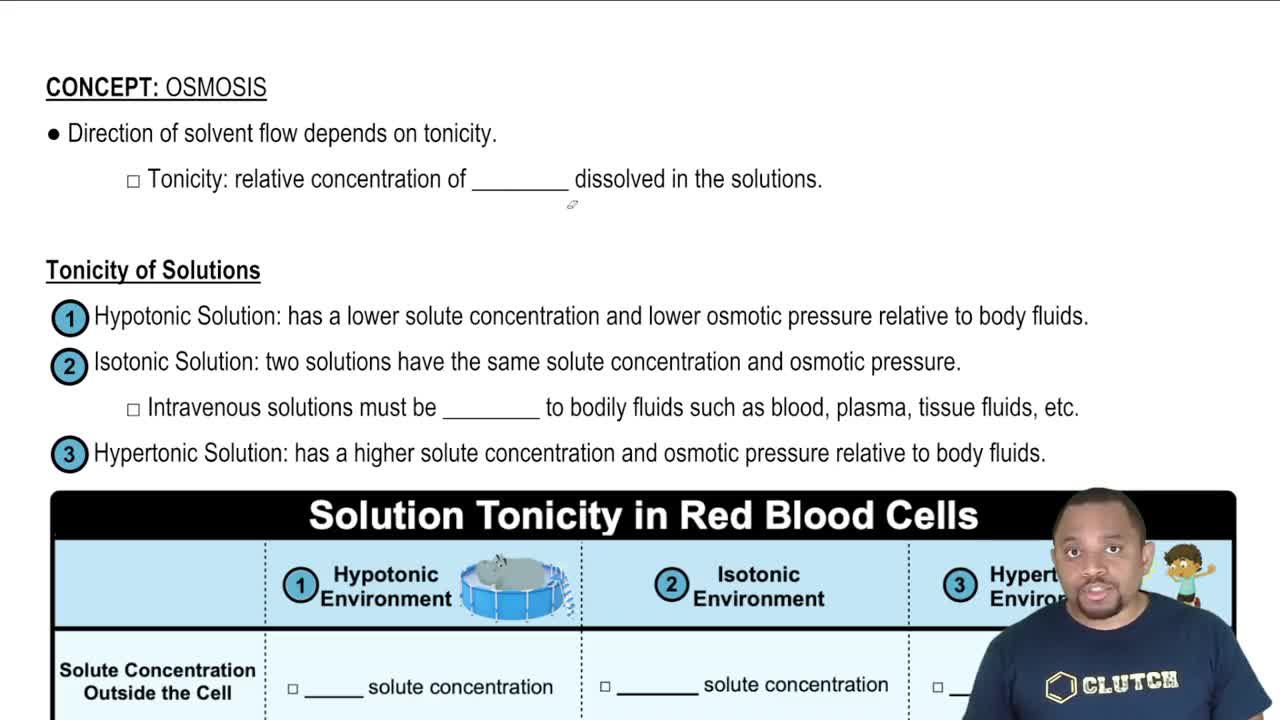
Osmolarity
Osmolarity is a measure of the total concentration of solute particles in a solution. It is expressed in osmoles per liter (osmol/L) and is crucial for understanding how solutions affect cell hydration and function. In the context of ORS, osmolarity helps determine the solution's effectiveness in rehydrating infants by comparing it to physiological levels found in blood plasma.
Recommended video:
Electrolytes
Electrolytes are minerals in the body that carry an electric charge and are essential for various physiological functions, including maintaining fluid balance and nerve transmission. In the ORS, sodium (Na⁺), potassium (K⁺), and chloride (Cl⁻) are key electrolytes that contribute to the osmolarity of the solution, influencing its ability to rehydrate effectively.
Recommended video:
Electrolytes (Simplified) Example 3
Comparison with Blood Plasma
Blood plasma has a typical osmolarity of about 280-300 mOsm/L, which is vital for maintaining homeostasis in the body. Comparing the osmolarity of the ORS to that of blood plasma helps assess whether the solution is isotonic, hypotonic, or hypertonic, which in turn affects its suitability for rehydration therapy in infants.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution

 1:43m
1:43m