Textbook Question
Show how ethylamine (C₂H₅NH₂) reacts with hydrochloric acid to form an ethylammonium salt.
584
views
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution
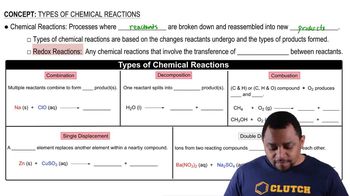
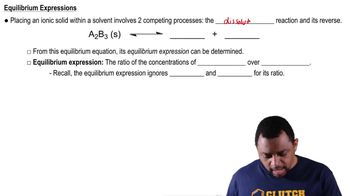

 3:08m
3:08mMaster Acid-Base Reactions Concept 1 with a bite sized video explanation from Jules Bruno
Start learning