Here are the essential concepts you must grasp in order to answer the question correctly.
Group Number
The group number in the periodic table indicates the column in which an element is located. Elements in the same group share similar chemical properties and have the same number of valence electrons. For example, barium is located in Group 2, which means it has similar characteristics to other alkaline earth metals.
Recommended video:
Calculate Oxidation Numbers
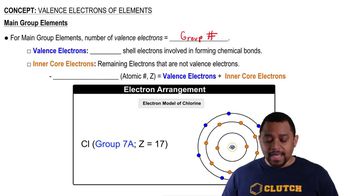
Valence Electrons
Valence electrons are the electrons in the outermost shell of an atom and are crucial for determining how an element reacts chemically. The number of valence electrons influences an element's bonding behavior and reactivity. Barium, being in Group 2, has two valence electrons, which it can lose to form positive ions.
Recommended video:
Valence Electrons of Elements (Simplified) Concept 1
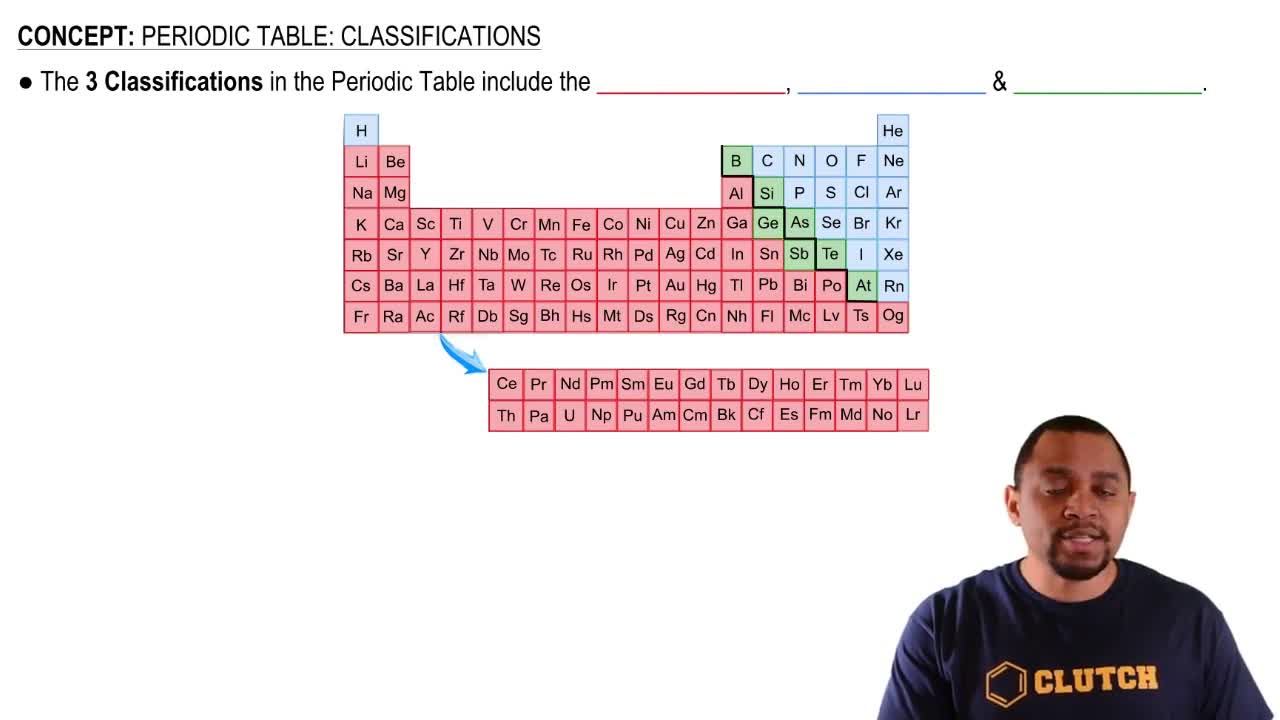
Periodic Table
The periodic table is a systematic arrangement of elements based on their atomic number, electron configuration, and recurring chemical properties. It is organized into rows (periods) and columns (groups), allowing for easy identification of an element's group number and its corresponding valence electrons. Understanding the layout of the periodic table is essential for predicting element behavior.
Recommended video:
Periodic Table: Classifications
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution

 1:55m
1:55m