Here are the essential concepts you must grasp in order to answer the question correctly.
Coenzymes
Coenzymes are organic molecules that assist enzymes in catalyzing biochemical reactions. They often act as carriers for chemical groups or electrons during these reactions. FAD (flavin adenine dinucleotide) is a well-known coenzyme that plays a crucial role in redox reactions, facilitating the transfer of electrons.
Recommended video:
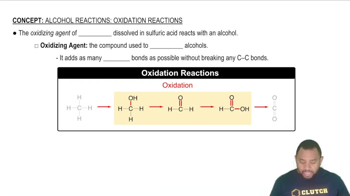
Oxidizing Agent
An oxidizing agent is a substance that gains electrons in a chemical reaction, causing another substance to be oxidized. In the context of redox reactions, the oxidizing agent is reduced itself. Understanding whether FAD acts as an oxidizing or reducing agent is essential for grasping its role in metabolic pathways.
Recommended video:
Alcohol Reactions: Oxidation Concept 1
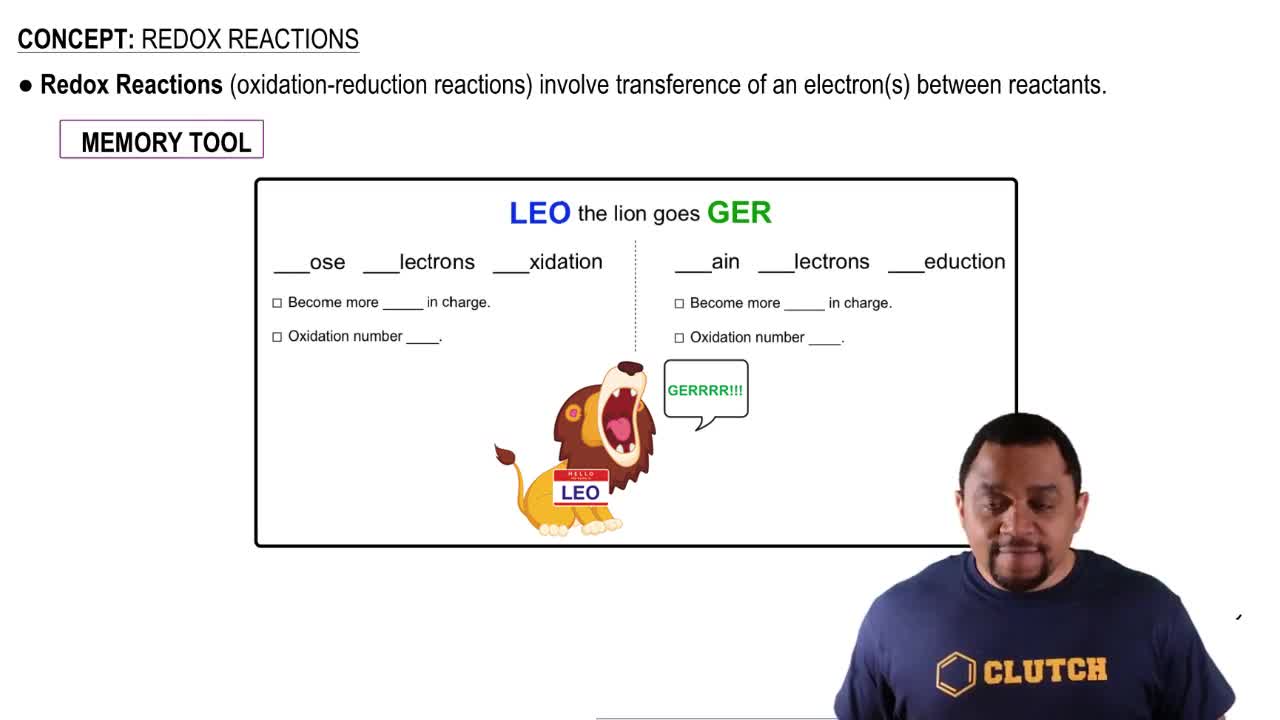
Redox Reactions
Redox reactions, short for reduction-oxidation reactions, involve the transfer of electrons between two substances. In these reactions, one substance is oxidized (loses electrons) while the other is reduced (gains electrons). FAD participates in these reactions by accepting electrons, thus functioning as an oxidizing agent.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution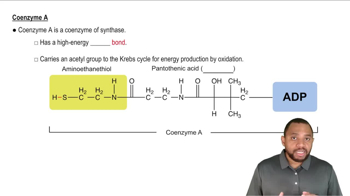

 2:2m
2:2m