Here are the essential concepts you must grasp in order to answer the question correctly.
Density
Density is defined as mass per unit volume, typically expressed in grams per milliliter (g/mL) for solids and liquids. It is a crucial property that helps identify materials, as different substances have unique densities. In this question, the densities of aluminum (2.70 g/mL) and silver (10.5 g/mL) are provided, allowing for the identification of the solids based on their mass and volume.
Recommended video:
Mass and Volume Relationship
The relationship between mass and volume is fundamental in determining density. For a given mass, the volume can be calculated using the formula: Volume = Mass / Density. In this scenario, since each solid has a mass of 10.0 g, the volume can be derived from the known densities, which will help in identifying each solid based on their respective volumes.
Recommended video:
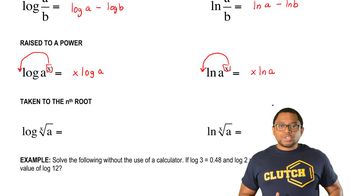
Logarithmic Relationships
Material Identification
Material identification involves using physical properties, such as density, to distinguish between different substances. By calculating the volume of each solid using their mass and known densities, one can match the calculated volumes to the expected volumes for aluminum and silver. This process is essential in determining which solid corresponds to which material in the given problem.
Recommended video:

 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution