Here are the essential concepts you must grasp in order to answer the question correctly.
Citric Acid Cycle
The citric acid cycle, also known as the Krebs cycle, is a series of enzymatic reactions that occur in the mitochondria, where acetyl-CoA is oxidized to produce energy. This cycle is crucial for cellular respiration, as it generates electron carriers like NADH and FADH₂, which are essential for the electron transport chain. Importantly, molecular oxygen is not directly involved in the cycle, but it is necessary for the subsequent oxidative phosphorylation process.
Recommended video:
Citric Acid Cycle Summary Concept 12
Hydride Ion Transfer
In biochemical reactions, hydride ions (H⁻) are often transferred from substrates to electron acceptors, playing a key role in oxidation-reduction reactions. In the citric acid cycle, NAD⁺ serves as the primary acceptor of hydride ions, converting to NADH. This transfer is vital for energy production, as NADH later donates electrons to the electron transport chain, ultimately leading to ATP synthesis.
Recommended video:
Proton Transfer
Proton transfer refers to the movement of hydrogen ions (H⁺) during biochemical reactions, which can influence pH and energy dynamics within cells. In the context of the citric acid cycle, FAD is the main acceptor of hydrogen ions, becoming FADH₂. This process is essential for maintaining the balance of redox reactions and contributes to the overall energy yield from the oxidation of acetyl groups.
Recommended video:
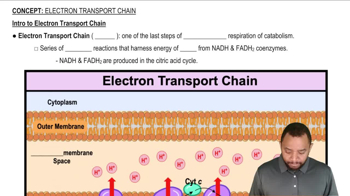
Intro to Electron Transport Chain Concept 1
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution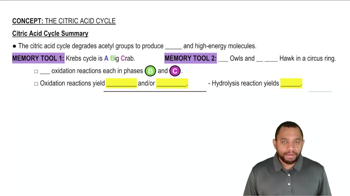


 :50m
:50m