Here are the essential concepts you must grasp in order to answer the question correctly.
Polyatomic Ions
Polyatomic ions are ions that consist of two or more atoms bonded together, carrying a net charge. They can be positively charged (cations) or negatively charged (anions). Understanding polyatomic ions is essential for naming and writing chemical formulas, as they often appear in various compounds.
Recommended video:
Sulfite Ion
The sulfite ion (SO₃²⁻) is a polyatomic ion composed of one sulfur atom and three oxygen atoms, with a net charge of -2. It is important in various chemical reactions and is often encountered in compounds like sodium sulfite. The bisulfite ion (HSO₃⁻) is derived from the sulfite ion by the addition of a hydrogen ion, resulting in a -1 charge.
Recommended video:
Naming Conventions
Naming conventions in chemistry provide systematic rules for naming compounds and ions. For polyatomic ions, prefixes and suffixes indicate the number of oxygen atoms and the charge. For example, 'bisulfite' refers to the HSO₃⁻ ion, indicating the presence of a hydrogen atom in the sulfite ion, which alters its charge and name.
Recommended video:
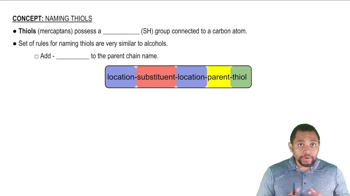
Rules for Naming Thiols Concept 1
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution