Here are the essential concepts you must grasp in order to answer the question correctly.
pH Scale
The pH scale measures the acidity or basicity of a solution, ranging from 0 to 14. A pH of 7 is neutral, while values below 7 indicate acidity and above 7 indicate alkalinity. The scale is logarithmic, meaning each whole number change represents a tenfold change in hydrogen ion concentration.
Recommended video:
Hydronium Ion Concentration
Hydronium ion concentration, represented as [H₃O⁺], indicates the amount of hydronium ions present in a solution. It is directly related to the acidity of the solution; higher concentrations correspond to lower pH values. In this case, a concentration of 4 x 10⁻² M indicates a significant level of acidity.
Recommended video:
Percent Concentrations Concept 1
Calculating pH
To calculate pH from hydronium ion concentration, the formula pH = -log[H₃O⁺] is used. This formula allows for the conversion of the molarity of hydronium ions into a pH value. For example, if [H₃O⁺] is 4 x 10⁻² M, the pH can be calculated by taking the negative logarithm of this concentration.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution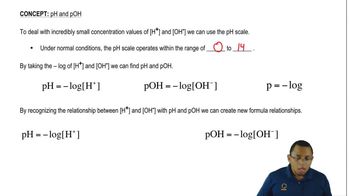



 1:31m
1:31m