Here are the essential concepts you must grasp in order to answer the question correctly.
Electronegativity
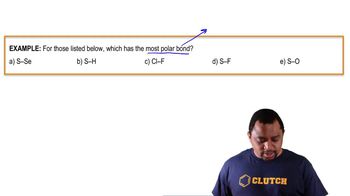
Electronegativity is a measure of an atom's ability to attract and hold onto electrons in a chemical bond. It is a key factor in determining the nature of the bond formed between two atoms. The higher the electronegativity, the stronger the atom's pull on shared electrons, influencing whether a bond is polar or nonpolar.
Recommended video:
Dipole Moment (Simplified) Concept 1
Covalent Bonds
Covalent bonds are formed when two atoms share one or more pairs of electrons. The nature of the bond—whether it is polar or nonpolar—depends on the difference in electronegativity between the bonded atoms. Nonpolar covalent bonds occur when the electronegativity difference is minimal, allowing for equal sharing of electrons.
Recommended video:
Electronegativity Difference
The electronegativity difference between two atoms determines the type of bond they will form. A difference of 0.0 to 0.4 typically indicates a nonpolar covalent bond, where electrons are shared equally. In contrast, larger differences lead to polar covalent or ionic bonds, where electron sharing is unequal or one atom completely transfers electrons to another.
Recommended video:
Dipole Moment (Simplified) Example 3
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 1:51m
1:51m