Here are the essential concepts you must grasp in order to answer the question correctly.
Acid-Catalyzed Hydrolysis
Acid-catalyzed hydrolysis is a chemical reaction where an ester reacts with water in the presence of an acid catalyst, typically a strong acid like sulfuric acid. This process breaks the ester bond, resulting in the formation of an alcohol and a carboxylic acid. The acid catalyst increases the reaction rate by protonating the carbonyl oxygen, making the carbon more electrophilic and susceptible to nucleophilic attack by water.
Recommended video:
Acid-Catalyzed Ester Hydrolysis Concept 1
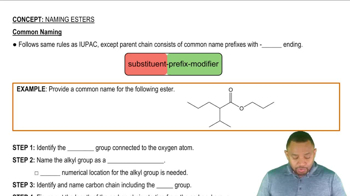
Esters
Esters are organic compounds formed from the reaction of an alcohol and a carboxylic acid, characterized by the functional group -COO-. They often have distinctive odors and are commonly found in fats, oils, and natural fragrances. In the context of hydrolysis, esters can be converted back into their parent alcohol and acid, which is essential for understanding the products formed during the reaction.
Recommended video:
Common Naming: Esters Concept 2
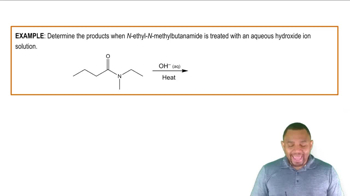
Products of Hydrolysis
The products of acid-catalyzed hydrolysis of an ester include an alcohol and a carboxylic acid. For isopropyl benzoate, the hydrolysis would yield isopropanol (the alcohol) and benzoic acid (the carboxylic acid). Understanding the structure of the starting ester is crucial for predicting the specific products formed during the hydrolysis reaction.
Recommended video:
Basic Hydrolysis Example 2
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution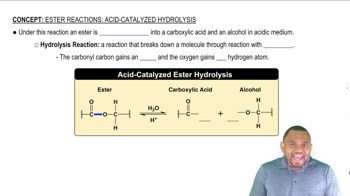

 1:14m
1:14m