Here are the essential concepts you must grasp in order to answer the question correctly.
Lewis Structures
Lewis structures are diagrams that represent the bonding between atoms in a molecule and the lone pairs of electrons that may exist. They help visualize the arrangement of valence electrons and the connectivity of atoms, which is crucial for predicting molecular geometry and reactivity. In the case of CF₄ and NF₃, their Lewis structures reveal the number of bonds and lone pairs, which influence their shapes.
Recommended video:
Lewis Dot Structures: Ions (Simplified) Concept 1
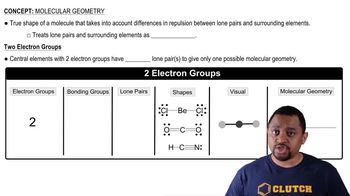
Molecular Geometry
Molecular geometry refers to the three-dimensional arrangement of atoms within a molecule. It is determined by the number of bonding pairs and lone pairs of electrons around the central atom, as described by the VSEPR (Valence Shell Electron Pair Repulsion) theory. CF₄ has a tetrahedral shape due to four bonding pairs and no lone pairs, while NF₃ has a trigonal pyramidal shape because of three bonding pairs and one lone pair.
Recommended video:
Molecular Geometry (Simplified) Concept 1
VSEPR Theory
VSEPR theory is a model used to predict the shape of molecules based on the repulsion between electron pairs surrounding a central atom. According to this theory, electron pairs will arrange themselves to minimize repulsion, leading to specific molecular geometries. This theory explains why CF₄ adopts a tetrahedral shape and NF₃ takes on a trigonal pyramidal shape, as the lone pair in NF₃ alters the spatial arrangement of the bonding pairs.
Recommended video: