Here are the essential concepts you must grasp in order to answer the question correctly.
Osmolarity
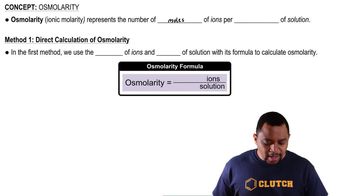
Osmolarity is a measure of the total concentration of solute particles in a solution. It is expressed in osmoles per liter (osmol/L) and accounts for all particles, including ions and molecules. For ionic compounds, osmolarity is calculated by considering the dissociation of the compound into its constituent ions.
Recommended video:
Dissociation of Ionic Compounds
Ionic compounds, such as KBr and K₂SO₄, dissociate into their respective ions when dissolved in water. For example, KBr dissociates into K⁺ and Br⁻ ions, while K₂SO₄ dissociates into 2 K⁺ and SO₄²⁻ ions. This dissociation increases the number of particles in solution, which is crucial for calculating osmolarity.
Recommended video:
Calculating Osmolarity
To calculate the osmolarity of a solution, multiply the molarity of each solute by the number of particles it produces upon dissociation. For instance, a 0.35 M KBr solution has an osmolarity of 0.35 M × 2 = 0.70 osmol/L, while a 0.15 M glucose solution remains 0.15 M (non-dissociating), and a 0.05 M K₂SO₄ solution has an osmolarity of 0.05 M × 3 = 0.15 osmol/L, leading to a total osmolarity for the mixture.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution