Here are the essential concepts you must grasp in order to answer the question correctly.
Lewis Symbols
Lewis symbols represent the valence electrons of an atom, depicted as dots around the element's symbol. They are crucial for understanding how atoms bond, as they illustrate the number of electrons available for bonding. In the context of elements X and Y, analyzing their Lewis symbols helps determine their bonding behavior, whether they will form ionic or molecular compounds.
Recommended video:
Lewis Dot Symbols (Simplified) Concept 2
Ionic vs. Molecular Compounds
Ionic compounds are formed through the transfer of electrons from one atom to another, typically between metals and nonmetals, resulting in charged ions. In contrast, molecular compounds are formed by the sharing of electrons between nonmetals. Understanding the nature of the elements involved (X and Y) and their electronegativity differences is essential to predict whether the compound will be ionic or molecular.
Recommended video:
Electronegativity
Electronegativity is a measure of an atom's ability to attract and hold onto electrons in a bond. The difference in electronegativity between two elements can indicate the type of bond they will form: a large difference typically leads to ionic bonding, while a small difference suggests covalent bonding. Evaluating the electronegativity of elements X and Y is key to determining the nature of their compound.
Recommended video:
Dipole Moment (Simplified) Concept 1

 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution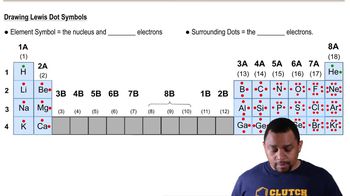



 1:10m
1:10m