Here are the essential concepts you must grasp in order to answer the question correctly.
Electron Configuration
Electron configuration describes the distribution of electrons in an atom's shells and subshells. The notation '2 8 18 8 2' indicates the number of electrons in each shell, which helps determine the element's position in the periodic table. Understanding this configuration is crucial for identifying the element's group, period, and properties.
Recommended video:
The Electron Configuration: Condensed
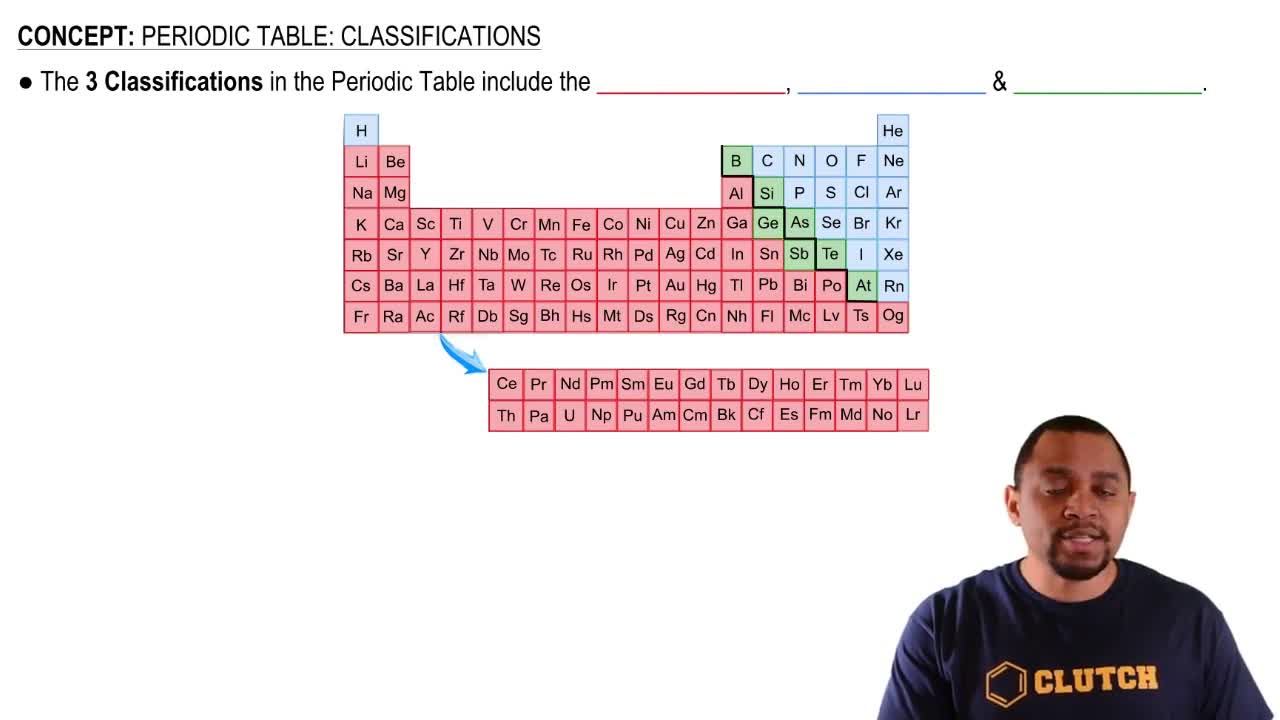
Periodic Table Organization
The periodic table organizes elements based on their atomic number, electron configuration, and recurring chemical properties. Groups (columns) indicate elements with similar properties, while periods (rows) represent elements with the same number of electron shells. This organization aids in predicting the element's behavior and classification as a metal or nonmetal.
Recommended video:
Periodic Table: Classifications
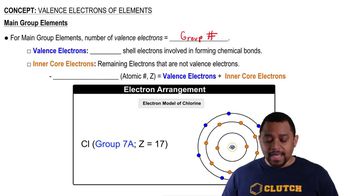
Valence Electrons and Electron-Dot Symbols
Valence electrons are the outermost electrons of an atom and play a key role in chemical bonding and reactivity. The electron-dot symbol, or Lewis dot structure, visually represents these valence electrons around the element's chemical symbol. This representation helps in understanding the element's bonding capabilities and its classification as a metal or nonmetal.
Recommended video:
Valence Electrons of Elements (Simplified) Concept 1
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution

 0:52m
0:52m