Here are the essential concepts you must grasp in order to answer the question correctly.
Chemical Reaction Types
Understanding the type of chemical reaction is crucial for predicting products. Common types include synthesis, decomposition, single replacement, and double replacement reactions. Each type follows specific patterns that dictate how reactants transform into products, influencing the final outcome of the reaction.
Recommended video:
Types of Chemical Reactions Concept 1
Reactants and Products
In a chemical reaction, reactants are the starting substances that undergo change, while products are the substances formed as a result. Identifying the chemical formulas and states of the reactants is essential for predicting the products accurately, as it helps in applying the correct stoichiometric relationships and reaction mechanisms.
Recommended video:
Solubility Product Constant (Ksp) Concept 2
Balancing Chemical Equations
Balancing chemical equations is a fundamental skill in chemistry that ensures the law of conservation of mass is upheld. This involves making sure that the number of atoms for each element is the same on both sides of the equation. Properly balanced equations not only help in predicting products but also in understanding the stoichiometry of the reaction.
Recommended video:
Balancing Chemical Equations (Simplified) Concept 1
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: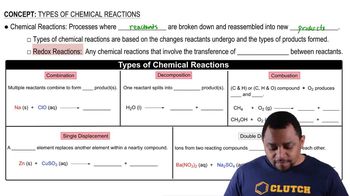


 1:3m
1:3m