Here are the essential concepts you must grasp in order to answer the question correctly.
Mutarotation
Mutarotation is the change in optical rotation that occurs when an anomeric carbon in a sugar ring opens and closes, interconverting between its alpha and beta forms. This process is significant in carbohydrates, as it affects the sugar's reactivity and properties. For example, in the case of galactose and fructose, understanding mutarotation helps predict how these sugars will behave in solution.
Recommended video:
Cyclic Forms of Sugars
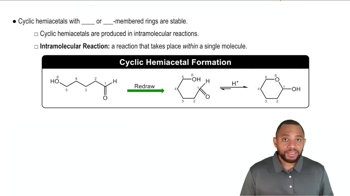
Cyclic forms of sugars, also known as hemiacetals or hemiketals, are the structures that sugars adopt when they form a ring. For instance, galactose can exist in both alpha and beta forms, depending on the orientation of the hydroxyl group at the anomeric carbon. Recognizing these cyclic structures is essential for understanding their chemical behavior and reactions, including mutarotation.
Recommended video:
Cyclic Hemiacetals Concept 2
Anomeric Carbon
The anomeric carbon is the carbon atom in a sugar that was originally part of the carbonyl group (aldehyde or ketone) and becomes a new chiral center when the sugar cyclizes. This carbon is crucial in determining the alpha or beta configuration of the sugar. In the context of mutarotation, the anomeric carbon's configuration influences the equilibrium between the cyclic forms of sugars like galactose and fructose.
Recommended video:
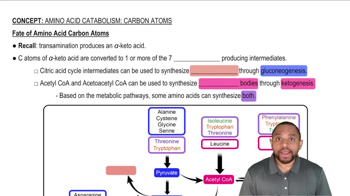
Amino Acid Catabolism: Carbon Atoms Concept 2
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution

 2:1m
2:1m