Here are the essential concepts you must grasp in order to answer the question correctly.
Periodic Table Groups
The periodic table is organized into columns called groups, which categorize elements based on similar chemical properties. Group 2A, also known as Group 2, contains alkaline earth metals, which are characterized by having two electrons in their outermost shell. Understanding the properties of elements in this group is essential for identifying their classification as metals.
Recommended video:
Periodic Table: Group Names
Metal Characteristics
Metals are typically characterized by their ability to conduct electricity and heat, malleability, ductility, and a shiny appearance. They tend to lose electrons during chemical reactions, forming positive ions. Elements in Group 2A are metals, specifically alkaline earth metals, which exhibit these properties and are reactive, especially with water.
Recommended video:
Periodic Trend: Metallic Character
Element Classification
Elements are classified into three main categories: metals, nonmetals, and metalloids. Metals are generally found on the left side and in the center of the periodic table, while nonmetals are located on the right. Metalloids possess properties of both metals and nonmetals and are found along the zig-zag line. Recognizing these classifications helps in understanding the behavior and reactivity of elements.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: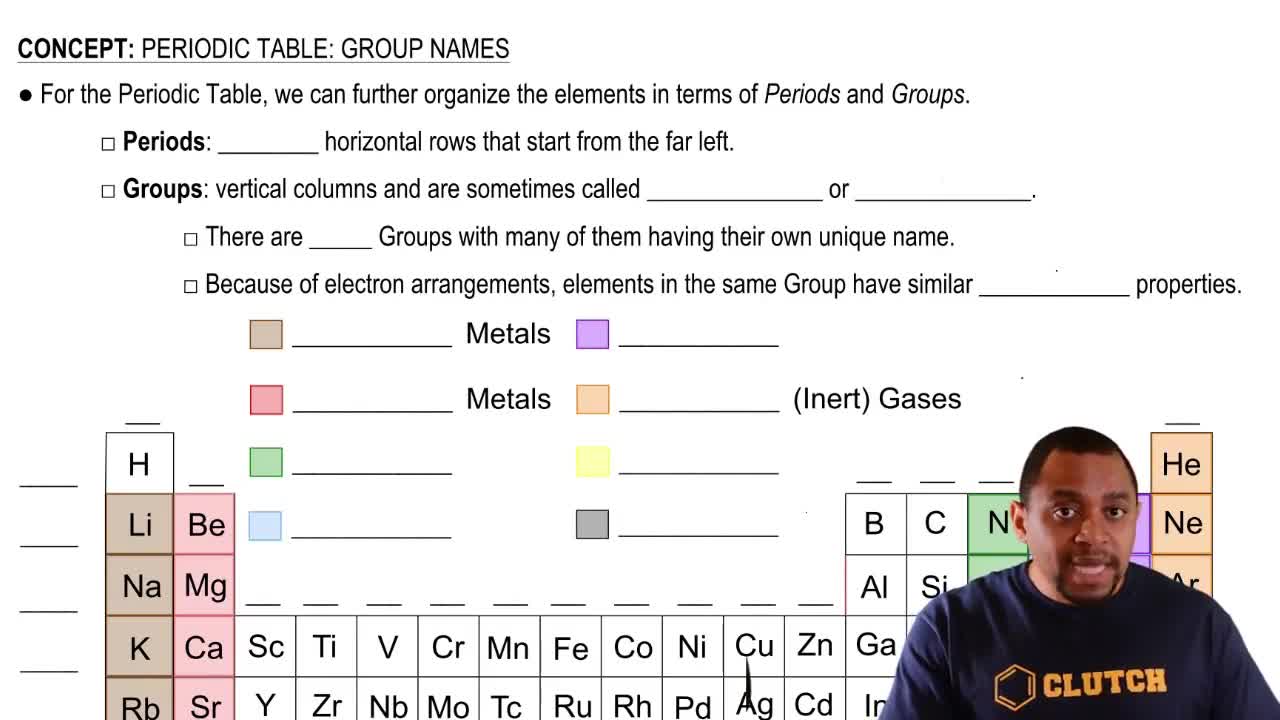


 2:40m
2:40m