Here are the essential concepts you must grasp in order to answer the question correctly.
Reduction and Oxidation
Reduction and oxidation are fundamental chemical processes that involve the transfer of electrons. Reduction refers to the gain of electrons or hydrogen, while oxidation involves the loss of electrons or hydrogen. In the context of the carbonyl group, the addition of a hydride ion (H⁻) signifies reduction, as it adds electrons to the carbonyl carbon, converting it to an alcohol.
Recommended video:
Reduction of Monosaccharides Example 1
Carbonyl Group
The carbonyl group (C=O) is a functional group characterized by a carbon atom double-bonded to an oxygen atom. It is a key feature in various organic compounds, including aldehydes and ketones. Understanding the structure and reactivity of the carbonyl group is essential for recognizing how it can be transformed through reduction and oxidation reactions.
Recommended video:
Functional Groups with Carbonyls Example 3
Hydride Ion (H⁻) and Proton (H⁺)
A hydride ion (H⁻) is a negatively charged ion consisting of a hydrogen atom with an extra electron, while a proton (H⁺) is a positively charged hydrogen ion. In reduction reactions, the hydride ion acts as a reducing agent, donating electrons to the carbonyl group. Conversely, the removal of H⁻ and H⁺ from an alcohol leads to the formation of a carbonyl group, illustrating the interplay between these species in redox chemistry.
Recommended video:
Ions (Simplified) Example 1
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: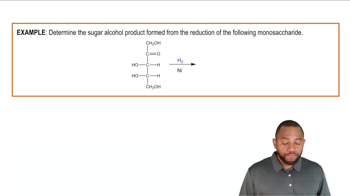


 2:38m
2:38m