Here are the essential concepts you must grasp in order to answer the question correctly.
Condensed Structural Formula
A condensed structural formula is a way of representing a chemical compound that shows the arrangement of atoms and the connectivity between them without depicting all the bonds explicitly. It typically groups atoms together to indicate how they are bonded, making it easier to visualize the structure of the molecule. For example, in a compound like C₄H₈O, the condensed formula would reflect the arrangement of carbon, hydrogen, and oxygen atoms in a compact form.
Recommended video:
Condensed Formula Concept 1
IUPAC Nomenclature
IUPAC nomenclature is a systematic method for naming chemical compounds based on their structure and functional groups. It provides a unique name that conveys information about the molecular structure, including the number of carbon atoms, the presence of functional groups, and the type of bonds. For the compound C₄H₈O, understanding IUPAC rules is essential to derive the correct name, which reflects its specific characteristics and classification.
Recommended video:
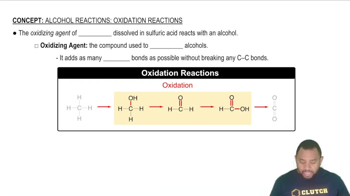
Oxidation of Alcohols
The oxidation of alcohols involves the conversion of alcohols into carbonyl compounds or carboxylic acids through the loss of hydrogen or the addition of oxygen. In the case of 2-methyl-1-propanol, oxidation leads to the formation of a carboxylic acid, which is a key aspect of understanding the reactivity of alcohols. This process is significant in organic chemistry as it helps in identifying the functional groups present in the resulting compounds.
Recommended video:
Alcohol Reactions: Oxidation Concept 1
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 1:57m
1:57m