Here are the essential concepts you must grasp in order to answer the question correctly.
Parts Per Million (ppm)
Parts per million (ppm) is a unit of measurement used to describe the concentration of a substance in a solution. It indicates how many parts of a solute are present in one million parts of the solution. For example, a concentration of 285 ppm means that there are 285 grams of solute in one million grams of solution, which is crucial for understanding how to dilute or mix solutions.
Recommended video:
Parts per Million (ppm) Concept 1
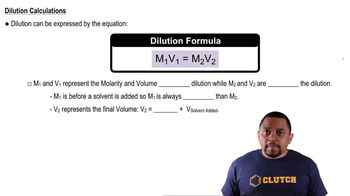
Dilution Principle
The dilution principle states that when a solution is diluted, the amount of solute remains constant while the volume of the solution increases. This can be mathematically expressed using the formula C1V1 = C2V2, where C1 and V1 are the concentration and volume of the initial solution, and C2 and V2 are the concentration and volume of the diluted solution. Understanding this principle is essential for calculating the required volumes to achieve a desired concentration.
Recommended video:
Concentration Calculation
Concentration calculation involves determining the amount of solute in a given volume of solution. In this context, it requires converting ppm to a more usable concentration unit, such as grams per liter, to facilitate calculations. This step is vital for accurately determining how much of the original solution is needed to achieve the target concentration in the final solution.
Recommended video:
Percent Concentrations Concept 1
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution